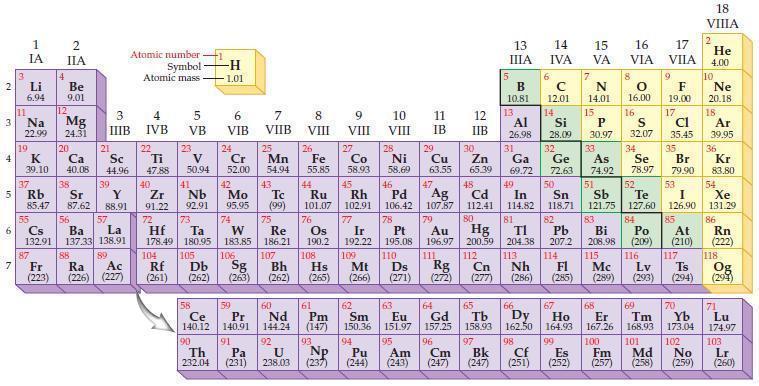
Refer to the periodic table and state the noble gas with an electron configuration identical to each
Question:
Refer to the periodic table and state the noble gas with an electron configuration identical to each of the following ions.
(a) S2–
(b) Cl–
(c) K+
(d) Ca2+.
Periodic Table
Transcribed Image Text:
2 3 4 5₁ 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55 4 87 2 IIA Fr (223) Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 38 Rb Sr Y 85.47 87.62 88.91 56 57 Cs Ba La 132.91 137.33 138.91 20 21 88 3 IIIB 39 89 Atomic number Symbol - Ac Ra (226) (227) Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 108 9 VIII 61 Pm (147) 27 Bh Hs Mt (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB Pt 195.08 110 29 Cu 63.55 13 IIIA 12 IIB 5 B 10.81 13 17 16 VA VIA VIIA 8 Al 26.98 6 C 12.01 14 Si 28.09 32 30 31 33 As Se Br Zn Ga Ge 65.39 69.72 72.63 74.92 78.97 79.90 14 15 IVA 7 N 14.01 15 P 30.97 16.00 83 Bi 208.98 115 16 S 32.07 34 84 47 48 49 50 51 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Au Hg 196.97 200.59 111 112 Rg Cn (271) (272) (277) (286) (285) (289) (293) (294) 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Mc Lv Ts Po (209) 9 116 F 19.00 Md (258) 17 Cl 35.45 35 53 I 126.90 85 At (210) 66 67 69 70 63 64 65 68 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 98 99 100 Cf Es Fm (251) (252) (257) 117 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Noble Gas for Each Ion a S2 Neon Ne Sulfur has 16 electronsand when gai...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the noble gas with an electron configuration identical to each of the following ions? (a) Se 2 (b) Br (c) Rb + (d) Sr 2+ . Periodic Table 2 3 4 5 6 7 3 11 Li...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) Br (b) Te 2 (c) As 3 (d) O 2 . Periodic Table: 2 3 4 10...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) F (b) S 2 (c) N 3 (d) I . Periodic Table: 2 3 4 10 6 3...
-
Marks 1. Find the limits, if they exist. If a limit does not exist, check whether the function approaches +00 x2 + 2x - 15 (5) (a) lim x-3 x2-4x +3 x2 - 4 (5) (b) lim x2 x4 - 16 Carol Ferland CF...
-
A firm emerging from Chapter 11 bankruptcy that does not qualify for fresh-start reporting must still report the effect of the reorganization plan on its financial position and results of operations....
-
Sydney, a single taxpayer, had $80,000 in adjusted gross income in year 2. During the year, she contributed $15,000 to her church. She also had a $17,000 contribution carryover from her year 1 church...
-
6. If growth is a significant value driver, does getting bigger translate into creating value? Explain.
-
Jetadiah Brown wants to establish a pet store, to be called Jets Pets. Jet thinks there is an opportunity in the south side of the city because he knows that many new subdivisions have been built and...
-
QUESTION 1 PT ABC issues bonds with a tenor of 10 years on January 1, 2021. The par value of the bonds is Rp. 1,500 per share with a total of 1,200 bonds issued. The coupon value of the bonds is 8%....
-
Using VSEPR theory, contrast the molecular shape of an ammonia molecule, NH3 , with that of an ammonium ion, NH4+.
-
Write out the electron configuration for each of the following nonmetal ions. (a) F (b) O 2 (c) N 3 (d) C 4 .
-
GDP does not count productive services, such as child care, food preparation, cleaning, and laundry, provided within the household. Why are these things excluded? Is GDP a sexist measure? Does it...
-
Q Proprietorinc (the lessee) enters into a 10 year lease of a property with an option to extend the contract for 5 years. Lease payments are $50,000 per year, payable at the beginning of each year....
-
1.Think about your investment Possibility for 3 years holding period in real investment environment? A.What could be your investment objectives? B. What amount of fund you could invest for three...
-
3- The student council normally sells 1500 school T-shirts for $12 each. This year they plan to decrease the price of the T-shirts. Based on student feedback, they know that for every $0.50 decrease...
-
2. The notation {f(x): x S} means "the set of all values that can be produced by substituting an element x of set S into f(x)." For example, the set of all odd integers can be expressed as {2k+1kZ}....
-
Implementation guidance for IFRS 2 indicates that it "accompanies, but is not part of, IFRS 2." In other words, this implementation guidance is considered mandatory. integral to the standard. not...
-
Consider Problem 13-18 involving Thomas Martin. Suggest when, if at all, the old should be replaced with the new, if the values for the old machine are as follows. The old machine retains only 70% of...
-
Define the essential properties of the following types of operating systems: a. Batch b. Interactive c. Time sharing d. Real time e. Network f. Parallel g. Distributed h. Clustered i. Handheld
-
Classification at costs, merchandising sector Home Entertainment Center (HEC) operates a large store in San Francisco. The store has both a video section and a music (compact disks and tapes)...
-
Classification of costs, manufacturing sector, the Fremont, California, plant of New United Motor Manufacturing, Inc. (NUMMI), a joint venture of General Motors and Toyota, assembles two types of...
-
Variable costs, fixed costs, total costs. Ana Compo is getting ready to open a small restaurant. She is on a tight budget and must choose between the following long-distance phone plans: Plan A: Pay...
-
Summarize in your own words Sharps, Treynors, and Jensens Measures for assessing portfolio performance with respect to risk. Assess the portfolio performance of mutual fund VDIGX taking into...
-
Question 1 Slat and Company have recently set up a business which will manufacture and sell a furniture component, the F12 On the 19 August 2021, the company issued 85,000 of share capital for cash....
-
The following is Addison Corporations contribution format income statements for last month. The company has no beginning or ending inventories. A total of 10,000 units were produced and sold last...

Study smarter with the SolutionInn App


