State the trends in the periodic table for decreasing atomic radii. Periodic Table: 2 3 4 10
Question:
State the trends in the periodic table for decreasing atomic radii.
Periodic Table:
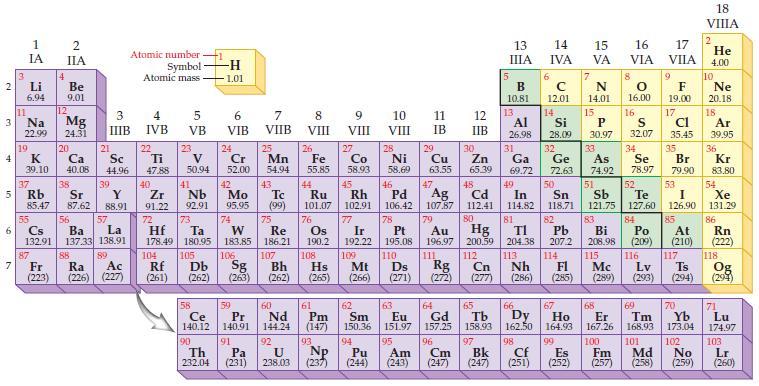
Transcribed Image Text:
2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 20 38 Ca Sc 40.08 44.96 21 Sr Y 85.47 87.62 88.91 55 56 La Cs Ba 132.91 137.33 138.91 88 3 4 IIIB IVB 39 57 89 Atomic number Symbol Atomic mass Ac Ra (226) (227) 22 Ti 47.88 5 VB 23 104 V 50.94 40 Nb Zr 91.22 92.91 41 105 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 106 Rf Db Sg (261) (262) (263) 59 Pr 140.91 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 27 Fe Co 55.85 58.93 Bh Hs (262) (265) 44 45 Ru Rh 101.07 102.91 76 Os 190.2 108 61 Pm (147) 9 VIII 93 U NP 238.03 (237) 77 Ir 192.22 109 Mt (266) 62 Sm 150.36 10 VIII 28 Ni 58.69 46 Pd 106.42 78 11 IB 94 95 Pu Am (244) (243) 13 IIIA 12 IIB 5 6 7 C 12.01 14 N 14.01 15 29 30 31 34 Si P 28.09 30.97 32 33 Cu Zn Ga Ge As Se 63.55 65.39 69.72 72.63 74.92 78.97 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 47 51 B 10.81 13 Al 26.98 14 15 IVA 16 VA VIA 79 81 82 Pt Au Hg TI Pb 195.08 196.97 200.59 204.38 207.2 110 111 112 113 114 Ds Rg Cn Nh Fl (271) (272) (277) (286) 83 Bi 208.98 115 16.00 16 52 S 32.07 84 Po (209) 17 VIIA 9 Md (258) F 19.00 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 116 Mc Lv (285) (289) (293) (294) Ts 117 66 70 63 64 65 67 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 101 102 Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The trends in the periodic table for decreasing atomic radii are as follows Atomic radii decrease from left to right across a period This is because t...View the full answer

Answered By

Rohail Amjad
Experienced Finance Guru have a full grip on various sectors, i.e Media, Insurance, Automobile, Rice and other Financial Services.
Have also served in Business Development Department as a Data Anlayst
4.70+
32+ Reviews
83+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
State the trends in the periodic table for decreasing metallic character. Periodic Table: 2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 20 38 Ca...
-
Table 9.2 does not include francium because none of franciums isotopes are stable. Predict the values of the entries for Fr in Table 9.2. Predict the nature of the products of the reaction of Fr...
-
Researching the current availability of multicore processors and which processors can be teamed in multiprocessor systems. Provide examples and insightful observations, such as benefits, cost,...
-
Simplify the expressions in Problems 3138. (3x - 1) (x + 3x - 2)
-
The FASAB sets standards for federal agencies that relate to external financial reporting, much like the GASB sets standards for state and local governments for external financial reporting. Do you...
-
Your physics study partner asks you to consider a parallel plate capacitor that has a dielectric completely filling the volume between the plates. He then claims that Eqs. (29.13) and (29.14) show...
-
Learn the success strategies for emerging markets. L01
-
What is just-in-time inventory management? What are its potential advantages?
-
Thank you very much for your assistance. Product or Period DM DL OH Selling Admin Cost Name Labor for assembly-line workers Salary for factory manager Depreciation for Factory Equipment Sales...
-
Predict the common ionic charge for Group IA/1 elements; Group IIA/2 elements; Group III/13 elements.
-
Predict the ionic charge for a chlorine ion, Cl ?- , based on the position of the element in the periodic table. Periodic Table: 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2 IIA 87 Be 9.01 12 K...
-
Dun Horse and Associates, a firm of real estate agents, had the following transactions represented during September: 1 Arranged a sale of an apartment building owned by a client. The commission for...
-
1) What are the benefits of home-based working for the company and the employees? 2) What are the challenges in performance management in working from home? 3) What is the right mix of office-based...
-
This assignment is focused on project selection and the underlying factors used to make this determination. You will need to use the readings/videos, the previous learning modules, along with some...
-
1. While improper framing could affect the information we have on sark attacks, I think our decisions come down to "anchoring and adjustment". Because the information we received from the media was...
-
For each of the scenarios in the following table, indicate the most likely reason for the difference in earnings. Scenario Differences in Human Capital Compensating Differential Differences in...
-
All organizations whether it is the government, a private business or small businessman require planning. To turn their dreams of increase in sale, earning high profit and getting success in business...
-
What is meant by a favorable labor efficiency variance? How is the labor efficiency variance computed?
-
As indicated by mutual fund flows, investors tend to beat the market seek safety invest in last year's winner invest in last years loser
-
On January 1, 2011, Irwin Animation sold a truck to Peete Finance for $33,000 and immediately leased it back. The truck was carried on Irwins books at $28,000. The term of the lease is 5 years, and...
-
Access the glossary (Master Glossary) to answer the following. (a) What is a bargain-purchase option? (b) What is the definition of incremental borrowing rate? (c) What is the definition of estimated...
-
What comprises a lessees minimum lease payments? What is excluded?
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


