State the trends in the periodic table for decreasing metallic character. Periodic Table: 2 3 4 10
Question:
State the trends in the periodic table for decreasing metallic character.
Periodic Table:
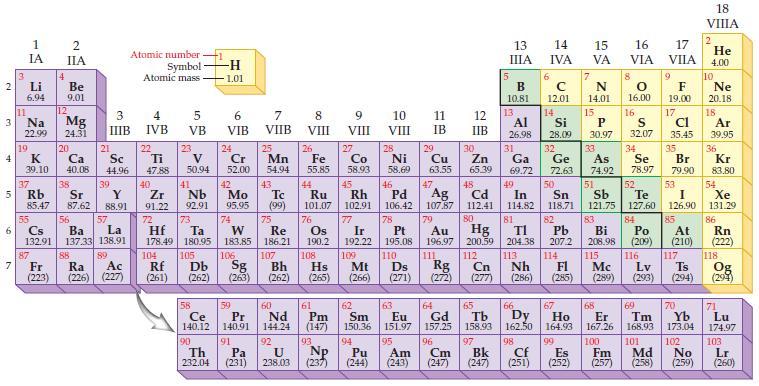
Transcribed Image Text:
2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 20 38 Ca Sc 40.08 44.96 21 Sr Y 85.47 87.62 88.91 55 56 La Cs Ba 132.91 137.33 138.91 88 3 4 IIIB IVB 39 57 89 Atomic number Symbol Atomic mass Ac Ra (226) (227) 22 Ti 47.88 5 VB 23 104 V 50.94 40 Nb Zr 91.22 92.91 41 105 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 106 Rf Db Sg (261) (262) (263) 59 Pr 140.91 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 27 Fe Co 55.85 58.93 Bh Hs (262) (265) 44 45 Ru Rh 101.07 102.91 76 Os 190.2 108 61 Pm (147) 9 VIII 93 U NP 238.03 (237) 77 Ir 192.22 109 Mt (266) 62 Sm 150.36 10 VIII 28 Ni 58.69 46 Pd 106.42 78 11 IB 94 95 Pu Am (244) (243) 13 IIIA 12 IIB 5 6 7 C 12.01 14 N 14.01 15 29 30 31 34 Si P 28.09 30.97 32 33 Cu Zn Ga Ge As Se 63.55 65.39 69.72 72.63 74.92 78.97 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 47 51 B 10.81 13 Al 26.98 14 15 IVA 16 VA VIA 79 81 82 Pt Au Hg TI Pb 195.08 196.97 200.59 204.38 207.2 110 111 112 113 114 Ds Rg Cn Nh Fl (271) (272) (277) (286) 83 Bi 208.98 115 16.00 16 52 S 32.07 84 Po (209) 17 VIIA 9 Md (258) F 19.00 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 116 Mc Lv (285) (289) (293) (294) Ts 117 66 70 63 64 65 67 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 101 102 Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Explanation Metallic character is increased from top to bottom down the group because electron...View the full answer

Answered By

Raghuram Suru
I am a Mechanical Engineer, I am an honest and hard working person, I stick to my commitments, I am professional in my dealings. I am a good learner of new things, that makes me good in problem solving. My key strength includes leadership, creativity, troubleshooting skills and quick problem solving.
I have wide knowledge in mechanical engineering field and 10 years of experience. I am willing to solve mechanical engineering problems and work related to mechanical projects.I am expert in following subjects.
Machine Design
Engineering Mechanics
Mechanics of Machines
Manufacturing
Thermodynamics
Heat And Mass Transfer
Mechanical Vibrations
Heat And Mass Transfer
Refrigerant and Air conditioning
Fluid Mechanics
Engineering Mathematics
You will be definitely satisfied with the my work.If you want to contact me any time as I am available for you in 24 hours.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Which statement is true about trends in metallic character? a) Metallic character increases as you move to the right across a row in the periodic table and increases as you move down a column. b)...
-
State the trends in the periodic table for decreasing atomic radii. Periodic Table: 2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 20 38 Ca Sc...
-
Table 9.2 does not include francium because none of franciums isotopes are stable. Predict the values of the entries for Fr in Table 9.2. Predict the nature of the products of the reaction of Fr...
-
Use Figure 5.25 to encode or decode the messages in Problems 3748. Multiply by 4 and add 10. WDVCTWDVGLR JR VCT RZFNTWT KZP.TY.
-
Describe the institutional process for establishing generally accepted accounting principles for the federal government.
-
A coil of wire wrapped on a hollow tube and a light bulb are connected in series to an ac source. What happens to the brightness of the bulb when an iron rod is inserted in the tube?
-
Do new global challengers pose any threat to firms from advanced economies? Explain. L01
-
The units of an item available for sale during the year were as follows: There are 50 units of the item in the physical inventory at December 31. The periodic inventory system is used. Determine the...
-
Polaski Company manufactures and sells a single product called a Ret. Operating at capacity, the company can produce and sell 32.000 Rets per year. Costs associated with this level of production and...
-
Predict the ionic charge for an aluminum ion, Al ?+ , based on the position of the element in the periodic table. Periodic Table: 2 3 4 15 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 Rb R 4 87 2 IIA Be...
-
Predict the ionic charge for a sodium ion, Na ?+ , based on the position of the element in the periodic table. Periodic Table: 2 3 4 5 6 7 Li 6.94 11 Na 22.99 19 1 IA K 39.10 37 55 4 2 IIA 87 Be 9.01...
-
Develop a list of references that will improve your employment prospects.
-
The Log Jamboree amusement park ride at Six Flags over Georgia consists of an approximately rectangular flume that is 6 ft wide and is constructed from fiberglass (ks = 0.002 in). In the low-velocity...
-
57'-8" 1. The building perimeter walls are 1'2" thick and the interior walls are 1'0" thick. Fig 1 and Fig 2 detail the linear feet of 1'2" -thick foundation walls. In addition, side B is 8'4" tall...
-
The Orpheus Chamber Orchestra is celebrating its 50 years as an orchestra this year. Read the following articles about its unique structure: The first Charlotte article is copied below, the rest just...
-
Which of the five strategies for adapting products and promotion for global markets does Monster Employ? 15-16. Which factors in the global marketing environment have challenged Monster's global...
-
Analysis of Current International Economic Environment in Switzerland 1. Develop a lead sentence for this section that introduces the key subsections 2. Economic Environment describe Switzerland...
-
Explain how efficiency and price or rate variances for direct labor can be closely interrelated.
-
Is it a breach of fiduciary duty for a director of a real estate investment trust (REIT) negotiating a joint venture on behalf of the REIT with another director for the development of a portfolio of...
-
On December 31, 2010, Shell hammer Co. sold 6-month old equipment at fair value and leased it back. There was a loss on the sale. Shell hammer pays all insurance, maintenance, and taxes on the...
-
The financial statements of P&G are presented in Appendix 5B or can be accessed at the books companion website, HYPERLINK "www.wiley.com/college/kieso" www.wiley.com/college/kieso. Refer to P&Gs...
-
Go to the books companion website or the company websites and use information found there to answer the following questions related to UAL, Inc. and Southwest Airlines. (a) What types of leases are...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


