If (E^{prime}) is the energy of elastically scattered neutron having incident energy (E) with a target nuclei
Question:
If \(E^{\prime}\) is the energy of elastically scattered neutron having incident energy \(E\) with a target nuclei of mass \(A\) in the LAB system, show that:
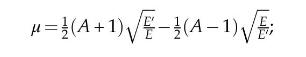
where \(\mu\) is the cosine of scattering angle in LAB system.
Transcribed Image Text:
={(A+1)(A1)E;
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)

Answered By

PRINCE PANDEY
I am Indian Chartered Accounting having a strong hold in the subjects of Accounting, IFRS Reporting, Indian
Taxation, Cost Accounting, Auditing. I have vast experience of teaching a student with easy way problem-solving approach.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A deuteron (nucleus of deuterium atom consisting of a proton and a neutron) with speed 14.9 km/s collides elastically with a neutron at rest. Use the approximation that the deuteron is twice the mass...
-
A simplified version of a commercial nuclear reactor involves fissile material such as enriched uranium 12 and a moderator such as graphite, both of which will be assumed in this exercise. Slow...
-
Show that the relativistic expression for the kinetic energy of a particle scattered through an angle ψ by a target particle of equal mass is The expression evidently reduces to Equation 9.89a in...
-
Doug Robinson and Dante are considering the possibility of opening their own manufacturing facility. They expect first-year sales to be $800,000, and they feel that their variable costs will be...
-
Cody's Cowboy Hat Emporium has two stores Fort Worth, TX. One in the Stockyards area that caters to tourists and another a mile further north that caters to ranch hands. Why doesn't Cody sell to both...
-
Pompeii Company sold $1,500,000, 12%, 10-year bonds on July 1, 2001. The bonds were dated July 1, 2001, and pay interest on July 1 and January 1. Pompeii Company uses the straight-line method to...
-
Outline some of the key stages to starting a fashion business. LO.1
-
Jerry Stevenson is the manager of a medical clinic in Scottsdale, AZ. He wants to analyze patient data to identify high-risk patients for cardiovascular diseases. From medical literature, he learned...
-
Question 1 Here is financial information for Sage Hill Inc.. December 31, 2020 Current assets $101.007 Plant assets (net) 367,488 Current liabilities 99,227 Long-term liabilities 126,268 Common stock...
-
Derive the expression for slowing down flux and slowing down density for an infinite homogeneous medium of purely hydrogenous medium with a source of strength \(S\). give a sketch of slowing down...
-
The geometric buckling of a bare cylinder of radius \(R\) and height \(H\) is given by \(B_{\mathrm{g}}^{2}=\) \((2.4 / R)^{2}+(\pi / H)^{2}\) using one group approximations. Show that the minimum...
-
a. Give the product(s) that would be obtained from the reaction of cis-2-butene and trans-2 butene with each of the following reagents. If the products can exist as stereoisomers, show which...
-
MTB Surfboards has a P / E of 2 0 . The discount rate for this firm is 3 0 percent. They had earnings of $ 2 , 0 0 0 , 0 0 0 and 1 0 0 , 0 0 0 shares of common stock outstanding. What should be the...
-
Question 4 (20 marks) Laboratory 4: Superposition Theorem Objectives: 1. Understand the principles of a Superposition Theorem 2. Determine the characteristics of a Superposition Theorem...
-
2 Ursala, Inc., has a target debt-equity ratio of .65. Its WACC is 10.4 percent, and the tax rate is 23 percent. a. If the company's cost of equity is 14 percent, what is its pretax cost of debt? b....
-
Thinking about Nike's corporate practices, discuss your approach to starting a company that outsourced labor in order to reduce manufacturing costs. What decisions would you make to combine...
-
Owen Properties recently purchased a building in a community that is eligible for participation in the National Flood Insurance Program (NFIP). Under the regular program of the NFIP, the maximum...
-
You have suspected for some time that production problems tend to flare up in the wintertime, during the first quarter of each year. A trend-seasonal analysis of the defect rates indicates seasonal...
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
Consider the structure of cyclopentadiene and then answer the following questions: (a) How many sp 3 -hybridized carbon atoms are present in the structure of cyclopentadiene? (b) Identify the most...
-
When (1R, 2R)-2-bromocyclohexanol is treated with a strong base, an epoxide (cyclic ether) is formed. Suggest a mechanism for formation of the epoxide: Strong base Br An epoxide
-
In the following reaction, determine whether the alkyne has been oxidized, reduced, or neither. Using the answer from the previous problem, try to determine the answer without calculating oxidation...
-
Jen bought 100 shares of ABC stock at $15 a share on July 14, 2017. On August 7, 2018, she noticed that the stock had increased in value to $20 a share and decided to sell her shares. Jen's marginal...
-
Alex. Inci, buys 40 petcent of Steinbart Company on January 1, 2020, for $1.212.000. The equity method of accounting is to be used. Steinbart's net assets on that datewere $2.90 million. Any excess...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2

Study smarter with the SolutionInn App


