When the following deuterium-labeled isomer undergoes elimination, the reaction yields trans-2-butene and cis-2-butene-2-d (as well as some
Question:
When the following deuterium-labeled isomer undergoes elimination, the reaction yields trans-2-butene and cis-2-butene-2-d (as well as some 1-butene-3-d ).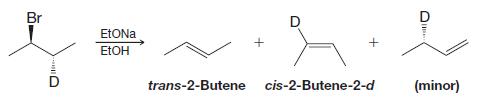
These compounds are not produced:
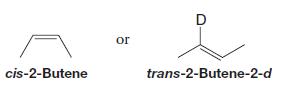
How can you explain these results?
Transcribed Image Text:
Br Ull EtONa EtOH trans-2-Butene श्र cis-2-Butene-2-d + D e (minor)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
The reaction appears to be an elimination reaction specifically an E2 reaction in which a molecule l...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
How can you explain the very different experiences of Maria and other teachers at the high school? Is there an avenue that could be taken to cope with these differences? How could this situation be...
-
How can you explain the fact that franc-i -hromo-2-methylcyclohcxane yields the non-Zaitsev?s elimination product 3-methylcyclohexene on treatment with base? H C Br trans-1-Bromo-2-methylcyclohexane...
-
How can you explain the huge disperity between bargaining coverage and union diversity in some countries? TABLE 12.2 Labor Relations around the Globe: Union Membership # Contract Coverage Country...
-
Answer the Multple Choice Questions and the code for problem 6in the end PROBLEM 1: General UNIX 1. What is UNIX? a) an operating system b) a text editor c) programming language d) software program...
-
1 / 2x 5/6 1/3 + 3/2 x Describe the solution set as an inequality, in interval notation, and on a graph.
-
Amanda and Raj are both students working part-time at an insurance company. Amanda can only work 5 hours a day. Her manager informs her that she needs to review 250 documents and process 250...
-
22. Assume Congress increases individual tax rates on ordinary income while leaving all other tax rates unchanged. How would this change affect the overall tax rate on corporate taxable income? How...
-
The widget industry is a constant-cost industry, so that all firms are identical. The following chart shows the industry-wide demand curve and the marginal cost curve of a typical firm: The industry...
-
Use the option quote information shown here to answer the questions that follow. The stock is currently selling for $73. Calls Puts Strike Option Expiration Price Vol. Last Vol. Last RWJ Mar 68 243...
-
Daniel B. Butler and Freida C. Butler, husband and wife, file a joint return. The Butlers live at 625 Oak Street in Corbin, KY 40701. Dan's Social Security number is 111-11-1112, and Freida's is...
-
Fluorination of (R)-2-fluorobutane yields a mixture of isomers with the formula C 4 H 8 F 2 . (a) How many different isomers would you expect to be produced? Write their structures. (b) If the...
-
There are nine stereoisomers of 1,2,3,4,5,6-hexachlorocyclohexane. Seven of these isomers are meso compounds and two are a pair of enantiomers. (a) Write structures for all of these stereoisomers,...
-
In a clinical trial to compare the effectiveness of two pain relievers, a sample of 100 patients was given drug 1 and an independent sample of 200 patients was given drug 2. Of the patients on drug...
-
For the data in Problem 42, how would you predict demand for medical kits using (a) moving averages and (b) exponential smoothing (with alpha values equal to 0.5 and greater) for the 21st week? Data...
-
For a light ray that crosses the interface between medium 1 having index of refraction \(n_{1}\) and medium 2 having index of refraction \(n_{2}\), what relationship between \(\theta_{1}\) and...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
Seniority quantum numbers typically measure how many fermions are in some sense "not paired" with another fermion. For the quasispin model of Problem 31.3 , define the Racah seniority $v$ through...
-
(a) Place a perfectly conducting sphere with radius a in a uniform electric field E 0 and let an origin centered electric dipole field represent the field produced by the sphere. Use this information...
-
Assume that a bank has assets located in London that are worth 150 million on which it earns an average of 8 percent per year. The bank has 100 million in liabilities on which it pays an average of 6...
-
3.16. For a system with non-identical service rates (see Sect. 3.5) and a limit of N jobs in the system (Eq. 3.13), obtain an expression for the mean service time per job, E[Ts], as a function of the...
-
Build a handheld model of bicycle [2.2.1] heptanes and discuss the types of strain that are present in this compound. udy tee op!
-
Explain whether the compound shown is the Z or the E diastereomer. Problems using online Three-Dimensional molecular models
-
Explain whether the conformation shown is the most stable conformation of each of these molecules. Problems using online Three-Dimensional molecular models
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


