Working backwards, deduce the starting material that led to the indicated product through the defined reactions. (a)
Question:
Working backwards, deduce the starting material that led to the indicated product through the defined reactions.
(a)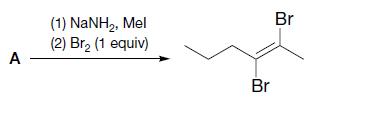
(b)
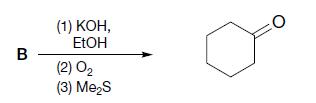
(c)
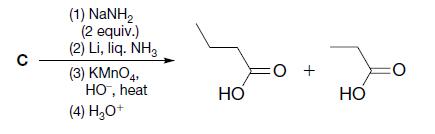
Transcribed Image Text:
Br (1) NaNH,, Mel (2) Br, (1 equiv) A - Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)

Answered By

Djordje Surdanovic
Chemistry seems like a boring, difficult, and arduous science, like other natural sciences. However, what if I told you that each of us has actually been a scientist since we were little? You must have wondered why the sun exists, why the water is liquid, the grass is green and the sky is blue? So did I, but never got the right answer explained in a suitable and interesting way. Therefore, I asked myself why couldn't I become an educator and try to improve and modify the long-existing school method. Just as my teen years began I took interest in guitar playing and was always fascinated by the tones. The vibrating of the strings made me wonder, what causes the sound? I then researched and explored that certain vibrations caused by the movement of atoms are responsible. Well, then I got only deeper and deeper into it, and eventually fell in love with chemistry. In my early university days, I started teaching at some private primary schools, and my method turned out pretty well. I then upgraded and moved on to give private lessons to my colleagues. A teaching assistant noticed me and offered me a job as a freelancer, or in other words an online tutor. And so did my freelance career started. I then applied for several companies and got my first job. Since then I have been helping students all around the globe! So are you afraid of chemistry? Well, let's fight that fear and finally understand chemistry as a part of our everyday life, not just as a science. Everyone is welcome!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Working backward, deduce the starting material that led to the indicated product through the defined reactions (A) (B) (1) NANH, (2 equiv) A (2) Li/NH,
-
Draw the structure of the starting material that would undergo azir-elimination give the -E isomer of the alkene product in the E2 reaction of Eq. 9.40.
-
The starting material for a commercial synthesis of vitamin C is l-sorbose (see the following reaction); it can be synthesized from d-glucose through the following reaction sequence: The second step...
-
Use (4.1), for a perfectly conducting fluid, and the nonlinear equation of continuity (1.1), to show that the change of B with time in a fluid element is related to changes of density according to...
-
Explain how each of the following is expected to affect growth: a. Increase in technology. b. Positive externalities. c. Patents. d. Learning by doing. e. Technological lock-
-
The current price of a stock is $20. It is expected to rise to $22 in one year and pay an annual dividend of $0.50 during the year. The RF is 5 percent; the ERM is 9 percent, and the stocks beta is...
-
Explain why the formula for sample variance is different from the formula for population variance.
-
Sirotka Retail Company began doing business in 2015. The following information pertains to its first three years of operation: Assume the following: The income tax rate is 40%. Purchase and sale...
-
Bramble Mills Limited follows IFRS, has a calendar year end, and adopted the policy of classifying interest paid as financing activities. It engaged in the following transactions in 2020. 1. The Land...
-
Enter the following transactions in the ledger of A Baker and prepare a trial balance at 31 May, together with a calculation of the profit for the month and a balance sheet at 31 May. May 1 Started...
-
In one industrial synthesis of ethanol, ethene first undergoes an addition reaction with sulfuric acid, and this product undergoes hydrolysis to ethanol. Write a mechanism for the addition of...
-
Given the following information, elucidate the structures of compounds A and B. Both compounds are soluble in dilute aqueous HCl, and both have the same molecular formula. The mass spectra of A and B...
-
In 2018, only 740,000 TopStuff meals were produced and sold to the hospitals. Smith suspects that hospital controllers had systematically inflated their 2018 meal estimates. 1. Recall that TopStuff...
-
how is lateral force(fy) determined from this data Tyre Responses 1 1 1 1 1 1.3 1.3 1.3 1.3 1.3 1.6 1.55 1.45 1.27 1.1 Fz (N) 0 400 800 1200 1500 Slip Angle (deg) Fy1 (N) Fy2 (N) Fy3 (N) 0.0 0 0 0.5...
-
(13%) Problem 8: A wire is oscillated to create a wave of the form y(x,t) = Asin(x - 30t) == The wave is reflected from a fixed end producing a reflection of the form y2(x,t) = A sin(x + 30t) The two...
-
Using the definitions of even integer and odd integer, give a proof by contraposition that this statement is true for all integers n: If 5n+3 is even, then n is odd.
-
7. Design the formwork for a wall 8-ft (2.44-m) high to be poured at the rate of 5 ft/h (1.53 m/h) at a temperature of 77F (25C). The concrete mixture will use Type I cement without retarders and is...
-
tempt in Progress The City of Minden entered into the following transactions during the year 2026. 1. A bond issue was authorized by vote to provide funds for the construction of a new municipal...
-
a. Which of the following items are within tolerance? b. What is the percent accuracy by item? Part Shelf Inventory Within Tolerance? Number Count Record Difference % Difference Tolerance 635 A 650...
-
Funds are separate fiscal and accounting entities, each with its own self-balancing set of accounts. The newly established Society for Ethical Teachings maintains two funds-a general fund for...
-
Using the information available in Figure 4.2, predict the position of the equilibrium in these reactions; that is, predict whether there is a higher concentration of reactants or products present at...
-
Use the information in Figure 4.2 to predict the positions of the equilibria in the reactions in problem 4.4.
-
Draw diagrams like that in Figure 4.3 for the reactions in problem 4.9.
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


