How can you explain the observation that the SN2 reaction of (dibromomethyl)benzene with NaOH yields benzaldehyde rather
Question:
How can you explain the observation that the SN2 reaction of (dibromomethyl)benzene with NaOH yields benzaldehyde rather than (dihydroxymethyl)benzene?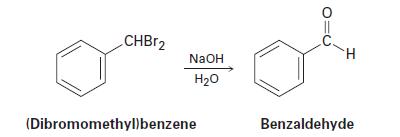
Transcribed Image Text:
CHBr2 NaOH H20 (Dibromomethylbenzene H Benzaldehyde
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
The observation that the SN2 reaction of bromomethylbenzene with NaOH yields benzaldehyde rather than hydroxymethylbenzene can be explained by the mec...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The SN2 reaction of (dihromomethyl) benzene, C6H5CHBr2, with NaOH yields benzaldehyde rather than (dihydroxymethyl) benzene, C6H5CH (0H)2 Explain.
-
Draw the product(s) obtained by conjugate addition of the following reagents to cyclohex-2-enone: (a) H 2 O (b) NH 3 (c) CH 3 OH (d) CH 3 CH 2 SH How can you explain the observation that the S N 2...
-
Aromatic compounds such as benzene react with alkyl chlorides in the presence of A1C1 3 catalyst to yield alkylbenzenes. The reaction occurs through a carbocation intermediate, formed by reaction of...
-
Solve each system. If a system is inconsistent or has dependent equations, say so. -5x + 2y = -4 6x + 3y = -6
-
Some companies that use process costing simply assign the entire cost of production to those units completed and transferred during the month, even if some units remain in process at the end of the...
-
Find a good window setting for the graph of the function. The graph should show all the zeros of the polynomial. f (x) = 3x 3 + 52x 2 - 12x - 12
-
The price of gold. Some people recommend that investors buy gold to protect against inflation. Here are the prices of an ounce of gold at the end of the year for the years between 1985 and 2007....
-
Idaho Food Processors processes potatoes into french fries. Production requires two processes: cutting and cooking. Direct materials are added at the beginning of the cutting process (potatoes) and...
-
Review the three real situations provided in this section and determine whether you believe that the entrepreneur mentioned in each scenario was being dishonest or simply resourceful. The scenarios...
-
Show the products that result from the reaction of phenylmagnesium bromide with the following reagents: (a) CH 2 O (b) Benzophenone (C 6 H 5 COC 6 H 5 ) (c) Pentan-3-one
-
Draw structures corresponding to the following names: (a) Bromoacetone (b) 3-Methylbutan-2-one (c) 3,5-Dinitrobenzaldehyde (d) 3,5-Dimethylcyclohexanone (e) 2,2,4,4-Tetramethylpentan-3-one (f)...
-
The Supreme Court of State A ruled that, under the law of State A, pit bull owners must either keep their dogs fenced or pay damages to anyone bitten by the dogs. Assess the accuracy of the following...
-
X 18. State the amplitude and period of: y = -4cos Graph one cycle of the function. 4 1 19. State the amplitude and period of: y = -sin(4x) Graph one cycle of the function. 4
-
Explain ways in which an organisation may overcome security vulnerabilities and issues?
-
A nonpipelined system takes 300ns to process a task. The same task can be processed in a 4-stage pipeline with a clock cycle of 50ns. Determine the speedup ratio of the pipeline for 400 tasks. What...
-
Within an orthodontic practice that I work in, insufficient patient care and poor time management are the most significant issues in the office. Beginning with the receptionists, scheduling...
-
How has the decision been improved with more of a focus on financial information? Why would it have been a better decision? How could you have included more financial information and where might it...
-
For the utility function U(q1, q2) = q1 + q2 , solve for the optimal q1 and q2?
-
Before the latest financial crisis and recession, when was the largest recession of the past 50 years, and what was the cumulative loss in output over the course of the slowdown?
-
Sigma bonds experience free rotation at room temperature: In contrast, Ï bonds do not experience free rotation. Explain. . cec - `H
-
Predict which of the following compounds is more acidic, and explain your choice. N- -N- -NH2 NH2
-
Consider the reaction below. The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the reaction mixture. The sodium iodide is not consumed by the reaction and...
-
Problem 12.6A (Algo) Liquidation of a partnership LO P5 Kendra, Cogley, and Mel share income and loss in a 3.21 ratio (in ratio form: Kendra, 3/6: Cogley, 2/6; and Mel, 1/6), The partners have...
-
Melody Property Limited owns a right to use land together with a building from 2000 to 2046, and the carrying amount of the property was $5 million with a revaluation surplus of $2 million at the end...
-
Famas Llamas has a weighted average cost of capital of 9.1 percent. The companys cost of equity is 12.6 percent, and its cost of debt is 7.2 percent. The tax rate is 25 percent. What is the companys...

Study smarter with the SolutionInn App


