Hydroxide ion reacts with chloromethane in a single step according to the following equation : +
Question:
Hydroxide ion reacts with chloromethane in a single step according to the following equation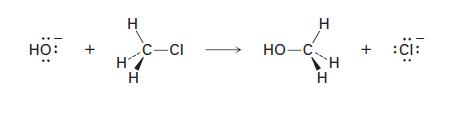
Transcribed Image Text:
но: + Н C-CI H/ Н НО-С Н Н H :CI:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
The reaction between hydroxide ion OH and chloromethane CHC...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
A) identify the limiting reagent and predict how many grams of sodium sulfate are produced when 2.0g of sulfur reacts with 3.0g of oxygen and 4.0g of sodium hydroxide according to the following...
-
Aluminum reacts with chlorine gas according to the following equation shown below. How many moles of Cl 2 are required to react with 0.11 mol of Al? 2 Al( s ) + 3 Cl 2 ( g ) 2 AlCl 3 ( s )
-
Perform the indicated operations. (3k + q)
-
As the manager of Smith Construction, you need to make a decision on the number of homes to build in a new residential area where you are the only builder. Unfortunately, you must build the homes...
-
In a transformer, an electric current in one coil of wire induces a current in a second coil. For a transformer, where i is the current and t is the number of windings in each coil. In a neon sign...
-
Classification and accumulation of costs by fixed and variable cost is of special importance in (a) service costing (b) job costing (c) contract costing (d) batch costing
-
Transactions related to revenue and cash receipts completed by Main Line Inc. during the month of August 2012 are as follows: Aug. 2. Issued Invoice No. 512 to Boston Co., $780. 4. Received cash from...
-
On January 1, 2012, the City of Austin issued 55,000,000 in mature in three years. The bonds have a stated rate of 8 percent and pay interest on June 30 and December 31 each year. When the bonds were...
-
If a reaction has E act = 15 kJ/mol, is it likely to be fast or slow at room temperature? Explain.
-
We said in Section 4.9 that an allylic carbocation is stabilized by resonance. Draw resonance structures to account for the similar stabilization of a benzylic carbocation. + CH A benzylic carbocation
-
O.J. Simpson was tried for murder both criminally and civilly. He was found innocent in the criminal trial but guilty in the civil trial. Why do you think that is the case?
-
From a square whose side has length \(x\), measured in inches, create a new square whose side is 5 in. longer. Find an expression for the difference between the areas of the two squares as a function...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A circle with radius 5
-
Find the present value of the ordinary annuities in Problems 21-32. Amount of Deposit m 23. $250 Frequency n semiannually Rate r 8% Time t 30 yr
-
Characterize the types of investments that are most vulnerable to political risk. Characterize those that are least vulnerable. What factors influence an investments vulnerability? On a scale of 1 to...
-
Refer to the following tree diagram for a two-stage experiment. Find the probabilities in Problems 1-6. \(P(B) \) E E A B C A B C
-
Use calculus to show that an increase in a specific sales tax ( reduces quantity less and tax revenue more, the less elastic the demand curve. (The quantity demanded depends on its price, which in...
-
Splitting hairs, if you shine a beam of colored light to a friend above in a high tower, will the color of light your friend receives be the same color you send? Explain.
-
Deduce the structure of a compound with molecular formula C 6 H 14 O 2 that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. 100 80 60 40 20 3000 2500 Wavenumber (cm-1) 4000 3500 2000 1500...
-
Deduce the structure of a compound with molecular formula C 6 H 14 O 2 that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. 100 80 60 40 20 4000 3500 3000 2500 2000 1500 1000 Wavenumber...
-
Deduce the structure of a compound with molecular formula C 8 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. 100 80 60 40 20 2500 2000 Wavenumber (cm-1) 1000 4000 3500 3000...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


