Reduction of butan-2-one with NaBH 4 yields butan-2-ol. Explain why the product is chiral but not optically
Question:
Reduction of butan-2-one with NaBH4 yields butan-2-ol. Explain why the product is chiral but not optically active.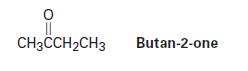
Transcribed Image Text:
CH3CCH₂CH3 Butan-2-one
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To understand why the product is chiral ...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain why the product obtained in the following reactions depends on the number of equivalents of base used in the reaction: 1. CHy cHj0en CH CCH2COCH CH 2 CH3Br one equivalet CHCCHCOCH2CH 1....
-
The federal government maintains a website devoted to providing information about product recalls. To complete this assignment, you will need to obtain information from this site as follows: Go to...
-
Reduction of 2-butanone with NaBH4 yields 2-butanol. Is the product chiral is it optically active? Explain.
-
In Exercises 1 through 22, find the critical points of the given functions and classify each as a relative maximum, a relative minimum, or a saddle point. f(x, y) = x 4 32x + y 3 12y + 7
-
Consider a two- player, sequential- move game where each player can choose to play right or left. Player 1 moves first. Player 2 observes player 1s actual move and then decides to move right or left....
-
The cross-sectional area A (in m 2 ) of a certain trapezoidal culvert in terms of its depth d (in m) is A = 2d + d 2 . Graph the possible values of d and A if A is between 1m 2 and 2m 2 .
-
A cinema hall has seating capacity of 800. It runs daily 4 shows on all 30 days of a month. It is generally full to the extent of 80% of its capacity. Find out the number of man shows during the...
-
For the coming year, Loudermilk Inc. anticipates fixed costs of $ 600,000, a unit variable cost of $ 75, and a unit selling price of $ 125. The maximum sales within the relevant range are $...
-
Q2: Muna and Baker Corporation is authorized to issue 50,000 shares of $5 par value common stock. During 2020, Lindsey Hunter took part in the following selected transactions. (10 Marks) 1. Issued...
-
Nonoxynol 9 is a potent spermicide made by reacting ethylene oxide with p-nonylphenoxide. Propose a mechanism for this multistep reaction. CH3(CH)8 p-Nonylphenoxide 0 Ethylene oxide CH3(CH)8...
-
Because all hamsters look pretty much alike, pairing and mating is governed by chemical means of communication rather than by physical attraction. Investigations have shown that dimethyl disulfide,...
-
Evaluate d/dt r(g(t)) using the Chain Rule. r(t) =(t 2 , t 3 ), g(t) = sin t
-
In 2020 the global distribution of sales in the industrial gas industry was as follows: i What is Air Liquides position on a GCI/GRI mapping? Global industrial gas industry 82 billion The 2020 global...
-
The General Social Survey polled a sample of 1048 adults in the year 2010, asking them how many hours per week they spent on the Internet. The sample mean was 9.79 with a standard deviation of 13.41....
-
An article in the Archives of Internal Medicine reported that in a sample of 244 men, 73 had elevated total cholesterol levels (more than 200 milligrams per deciliter). In a sample of 232 women, 44...
-
Explain how search can be used to solve constraint satisfaction problems, such as the eight-queens problem. What difficulties arise when such problems become extremely large (e.g., the...
-
Casse (1981) developed an exercise to encourage his students to develop their empathic skills. He asked them to listen to a recording of a dialogue between John Miller (a US project manager in...
-
Due to a recession that lowered incomes, the 2008 market prices for last-minute rentals of U.S. beachfront properties were lower than usual. Suppose that the inverse demand function for renting a...
-
SBS Company have received a contract to supply its product to a Health Care Service Hospital. The sales involve supplying 1,250 units every quarter, the sales price is RM 85 per unit. The Client...
-
Identify the reagents you would use to prepare each of the following compounds via a Diels-Alder reaction: (a) (b) (c) (d) (e) (f) (g) (h) COOH COOH
-
Starting with 1,3-butadiene as your only source of carbon atoms, and using any other reagents of your choice, design a synthesis of the following compound:
-
The following triene reacts with excess maleic anhydride to produce a compound with molecular formula C 14 H 12 O 6 . Draw the structure of this product (ignoring stereochemistry). Maleic anhydride...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


