Tetramethyloxirane is too hindered to undergo nucleophilic substitution by the hindered alkoxide, potassium tert butoxide. Instead, the
Question:
Tetramethyloxirane is too hindered to undergo nucleophilic substitution by the hindered alkoxide, potassium tert butoxide. Instead, the product is the allylic alcohol shown. Propose a mechanism to explain this reaction. What type of mechanism does it follow?
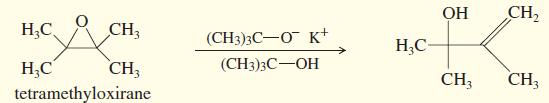
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





