The following tertiary alkyl bromide does not undergo a nucleophilic substitution reaction by either SN1 or SN2
Question:
The following tertiary alkyl bromide does not undergo a nucleophilic substitution reaction by either SN1 or SN2 mechanisms.Explain.
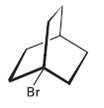
Transcribed Image Text:
Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
The first step in an Syl displacement is dissociati...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following tertiary alkyl bromides undergo an SN1 reaction in aqueous acetone to form the corresponding tertiary alcohols. List the alkyl bromides in order of decreasing reactivity. Br CH CCH3...
-
Show how you might use a nucleophilic substitution reaction of 1-bromopropane to synthesize each of the following compounds. (You may use any other compounds that are necessary.) (a) (b)...
-
Furfuryl chloride can undergo substitution by both SN2 and SN1 mechanisms. Since it is a 1° alkyl halide, we expect SN2 but not SN1 reactions. Draw a mechanism for the SN1 reaction shown below,...
-
Complete your review of the Stevens District Hospital Strategic Planning Scenario, you have been asked to provide a presentation to the governing board of the hospital. This board is comprised of the...
-
Although the board of directors is responsible for filling the CEO position, how could HR managers support the board with a succession management program?
-
The accounting staff of CCB Enterprises has completed the financial statements for the 2014 calendar year. The statement of income for the current year and the comparative statements of financial...
-
Describe commonly used appraisal methods.
-
Tell whether each of the following transactions related to an office building is a revenue expenditure (RE) or a capital expenditure (CE). In addition, indicate whether each transaction is an...
-
Exercise 11.24 DEPRECIATION CALCULATION Osamu Ltd operates a factory that contains a large number of machines designed to produce knitted garments. These machines are generally depreciated at 10%...
-
Refer to the projected financial statements for Massachusetts Stove Company (MSC) prepared for Case 10.2. The management of MSC wants to know the equity valuation implications of not adding gas...
-
In light of your answer to problem 11.42, what product might you expect from treatment of 4-hrorno-l-butanol withbase? Base BrCH2CH2CH2CH2
-
In addition to not undergoing substitution reactions, the alkyl bromide shown in Problem 11 .45 also fails to undergo an elimination reaction when treated with base. Explain.
-
What does it mean to say that the linear correlation coefficient between two variables equals 1? What would the scatter diagram look like?
-
Waverly Company Ltd. currently produces 8,000 units per year of SB 200 (snowboard), which is a component of the company's major products. SB 200 has the following unit cots Direct materials - $35.50...
-
Norton Ltd manufactures a single product, which is sold for $150 per unit. The standard variable costs per unit of the product are: Direct material 4 kilos at $8 per kilo Direct labour 5 hours at $10...
-
QUESTION 4 Murni Selasih Bhd is considering investing in a project that will generate higher returns Currently, the company has two projects with forecasted outcomes under consideration. The possible...
-
ABC plans to sell 60,000 units of product 751 in June, and each of these units requires five sq. ft. of raw material. Additional data is as follows: Product Raw No. 751 Material Actual June 1 11,200...
-
Case: Tom has felt anxious and constantly on edge over the past 3 years. He has few social contacts because of his nervous symptoms. He is married with 3 children and worries about if he is a good...
-
explain the logical basis of the accounting equation and why the balance sheet must balance;
-
As of January 1, 2018, Room Designs, Inc. had a balance of $9,900 in Cash, $3,500 in Common Stock, and $6,400 in Retained Earnings. These were the only accounts with balances in the ledger on January...
-
Calculate K p for each reaction. a. NO4(8) = 2 NO(g) b. N(g) + 3 H(g) = 2 NH3(g) c. N(g) + O(g) 2 NO(g) K = 5.9 x 10- (at 298 K) K = 3.7 x 108 (at 298 K) K = 4.10 x 10-1 (at 298 K)
-
Propose a mechanism for the reaction of cyclohexyl methyl ketone with excess bromine in the presence of sodium hydroxide.
-
Predict the products of the following reactions. (a) Cyclopentyl methyl ketone + excess Cl2 + excess NaOH (b) 1-cyclopentylethanol + excess I2 + excess NaOH (c) Propiophenone + excess Br2 + excess...
-
Which compounds will give positive iodoform tests? (a) 1-phenylethanol (b) Pentan-2-one (c) Pentan-2-ol (d) Pentan-3-one (e) Acetone (f) Isopropyl alcohol
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


