In light of your answer to problem 11.42, what product might you expect from treatment of 4-hrorno-l-butanol
Question:
In light of your answer to problem 11.42, what product might you expect from treatment of 4-hrorno-l-butanol withbase?
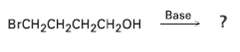
Transcribed Image Text:
Base BrCH2CH2CH2CH2он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
Methoxide removes a proton from the hydrox...View the full answer

Answered By

Sagar Kumar
I am Mechanical Engineer with CGPA of 3.98 out of 4.00 from Pakistan. I went to Government Boys Degree College, Sehwan for high school studies.
I appeared in NUST Entrance Exam for admission in university and ranked #516. My mathematics are excellent and I have participated in many math competitions and also won many of them. Recently, I participated in International Youth Math Challenge and was awarded with Gold Honor. Now, I am also an ambassador at International Youth Math Challenge,
I have been teaching when I was in 9th class class year 2012. I have taught students from 6th class to university level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In light of your answer to Problem 11.61, explain why one of the following isomers undergoes E2 reaction approximately 100 times as fast as the other. Which isomer is more reactive, andwhy? (a) CI...
-
In light of your answer to Problem 4.44, draw the two chair conformations of 1, 1, 3-trimcthylcyclohexanc, and estimate the amount of strain energy in each. Which conformation is favored?
-
In light of your answer to Problem 11.49, which alkene, F or Z, would you expect from an E2 reaction on the tosylate of (2R, 3R)-3-phenyl-2-butanol? Which alkene would result from E2 reaction on the...
-
With reference to the Auditors Report, answer the following questions: The recent final audit report of an FMCG carries a line which is read as in the manner so required and respectively give a true...
-
Based on the information given, what developmental approaches were part of McDonald's career development? What approaches would you recommend for preparing P&G's next top executives?
-
Comparative balance sheets for Farinet Company for the past two years are as follows: Required 1. Using the format in Example 13-4, prepare common-size comparative balance sheets for the two years...
-
Discuss the role of employee counseling in the appraisal process.
-
Draw a context diagram, a level 0 DFD, and a set of level 1 DFDs (where needed) for the campus housing use cases that you developed for the Your Turn 4-1 box in Chapter 4.
-
help please Minimum Deposit Term Length APY Average Percentage Yield Interest Rate 3.5% 4% 4% $10,000 $10,000 $10,000 12 months 18 months 39 months Withdrawals made after the first 7 days of account...
-
On February 12, 2002, Nancy Trout and Delores Lake formed Kingfisher Corporation to sell fishing tackle. Pertinent information regarding Kingfisher is summarized as follows: Kingfisher's business...
-
Show the stereochemistry of the epoxide you would obtain by formation of a bromohydrin from trans-2-butene, followed by treatment with base.
-
The following tertiary alkyl bromide does not undergo a nucleophilic substitution reaction by either SN1 or SN2 mechanisms.Explain. Br
-
Dynamic Energy Systems stock is currently trading for \($31\) per share. The stock pays no dividends. A one-year European put option on Dynamic with a strike price of \($41\) is currently trading for...
-
Given below is some is a comparison of financial performance data of a project when flexibility is incorporated (I.e. flexible project) in comparison to when it is not. (i.e. inflexible project) The...
-
For Service Zone H, assuming your shipment chargeable weight is between 100 and 300 kg, at what weight does it become cheaper to declare the shipment weight to be 300 kg.? EG: What is the rate break...
-
Gold Dust Ltd has produced the following budgeted data for its current financial year:- Sales 2900000 Direct materials 400000 Direct labour 500000 Production overhead 1200000 Production cost 2100000...
-
Critical Review V Hide Assignment Information Instructions Williams, A. (2012). Worry, intolerance of uncertainty, and statistics anxiety. Click on the following link to retrieve the article....
-
(4.) Octopussy Company uses a predetermined overhead rate in applying overhead to production orders on a labor-cost basis for Dept. A and on a machine-hour basis for Dept. B. At the beginning of...
-
explain what is meant by an 'entity' and why we account for its transactions separately from those of its owner(s);
-
Digital Fruit is financed solely by common stock and has outstanding 25 million shares with a market price of $10 a share. It now announces that it intends to issue $160 million of debt and to use...
-
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products. a. CO3- (aq) + HO(1) b. 2 KCIO3(s) = 2 KCl(s) + 3 O(g) c. HF(aq) + HO(1) H3O+...
-
Phenylacetone can form two different enols. (a) Show the structures of these enols. (b) Predict which enol will be present in the larger concentration at equilibrium. (c) Propose mechanisms for the...
-
An enolate is a very strong nucleophile. Bromine is a strong electrophile, so it can react with much weaker nucleophiles. Give mechanisms for the reactions of bromine with cyclopentene and with...
-
Propose a mechanism to show how acetophenone undergoes base-promoted chlorination to give trichloroacetophenone.
-
Answer please, A company uses the perpetual inventory system and recorded the following entry: This entry reflects a
-
As a Financial Analyst in the Finance Department of Zeta Auto Corporation they are seeking to expand production. The CFO asks you to help decide whether the firm should set up a new plant to...
-
Chapter 4 When an Auditor finds misstatements in entities financial statements which may be the result of fraudulent act, what should be the role of an auditor under that situation? (2 Points)

Study smarter with the SolutionInn App


