In light of your answer to Problem 11.61, explain why one of the following isomers undergoes E2
Question:
In light of your answer to Problem 11.61, explain why one of the following isomers undergoes E2 reaction approximately 100 times as fast as the other. Which isomer is more reactive, andwhy?
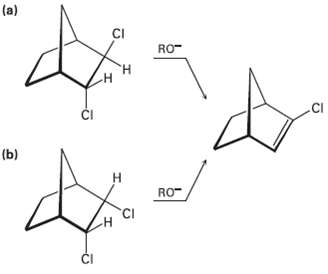
Transcribed Image Text:
(a) CI RO- н .CI ČI (b) Н RO- CI ČI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 59% (22 reviews)
H N H H A B We concluded in Problem 1161 that E2 elimination i...View the full answer

Answered By

Ashish Jaiswal
I have completed B.Sc in mathematics and Master in Computer Science.
4.90+
20+ Reviews
39+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In light of your answer to Problem 14.43, propose a mechanism for the followingreaction: CH CH + CO2 Heat "CCH do,c, H a-Pyrone CHC-
-
In light of your answer to problem 11.42, what product might you expect from treatment of 4-hrorno-l-butanol withbase? Base BrCH2CH2CH2CH2
-
In light of your answer to Problem 4.44, draw the two chair conformations of 1, 1, 3-trimcthylcyclohexanc, and estimate the amount of strain energy in each. Which conformation is favored?
-
Adam Kleen Enterprise (AKE) was initially set up as a convenient shop selling laundry related items by Adam Bollan. Despite having good results in the national-level examination and was even offered...
-
How could a well-designed training program help make this idea meet ethical as well as legal standards?
-
Make use of the data in Sample Problem 3- to describe the integration that would take place when the following transaction is entered into the computer: PaidDELTA Appliances, Inc., $1,500.00, less 2%...
-
Oktoberfest, The Carnival in Rio de Janeiro, Brazil, Reggae on the River, Mardi Gras, and the Grand Ole Opry are some of the larger fairs, festivals, and events. LO.1
-
The adjusted trial balance for Lloyd Construction as of December 31, 2020, follows: An analysis of other information reveals that Lloyd Construction is required to make a $41,500 payment on the...
-
Answer Instruction The following account appears in the ledger after only part of the postings have been completed for July, the fest month of the current fiscal year July 1 Work in Process Balance...
-
A. Richard McCarthy (born 2/14/64; Social Security number 100-10-9090) and Christine McCarthy (born 6/1/1966; Social security number 101-21-3434) have a 19-year-old son (born 10/2/99 Social Security...
-
Although anti periplanar geometry is preferred for E2 reactions, it isn?t absolutely necessary. The deuterated bromo compound shown here reacts with strong base to yield an un-deuterated alkene....
-
There are eight diastereomers of 1, 2, 3, 4, 5, 6-hexachlorocyclohexane. Draw each in its more stable chair conformation. One isomer loses HCI in an E2 reaction nearly 1000 times more slowly than the...
-
A football quarterback shows off his skill by throwing a pass 45.70 m downfield and into a bucket. The quarterback consistently launches the ball at 38.00 above horizontal, and the bucket is placed...
-
X 18. State the amplitude and period of: y = -4cos Graph one cycle of the function. 4 1 19. State the amplitude and period of: y = -sin(4x) Graph one cycle of the function. 4
-
Explain ways in which an organisation may overcome security vulnerabilities and issues?
-
A nonpipelined system takes 300ns to process a task. The same task can be processed in a 4-stage pipeline with a clock cycle of 50ns. Determine the speedup ratio of the pipeline for 400 tasks. What...
-
Within an orthodontic practice that I work in, insufficient patient care and poor time management are the most significant issues in the office. Beginning with the receptionists, scheduling...
-
How has the decision been improved with more of a focus on financial information? Why would it have been a better decision? How could you have included more financial information and where might it...
-
prepare financial statements from a trial balance, including adjustments in respect of accruals (accrued expenses) and prepayments (prepaid expenses).
-
The comparative statements of financial position of Menachem NV at the beginning and end of the year 2019 appear below. Net income of ¬34,000 was reported, and dividends of ¬23,000 were paid...
-
Many equilibrium calculations involve finding the equilibrium concentrations of reactants and products given their initial concentrations and the equilibrium constant. Outline the general procedure...
-
Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with pyrrolidine.
-
1. Rank the following compounds in order of increasing acidity. 2. Indicate which compounds would be more than 99% deprotonated by a solution of sodium ethoxide in ethanol. () (b) () (d) () (f)...
-
Predict the products of the following aldol condensations. Show the products both before and after dehydration. (a) (b) (c) (d) (e) (f) CH3 TOH CH CH2-C-H CH TOH Ph-C-CH+ OH
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


