(a) Explain why ionization of a electron requires less energy than ionization of a electron....
Question:
(a) Explain why ionization of a π electron requires less energy than ionization of a σ electron.
(b) Draw the structure of the molecular ion of 1-heptene formed by ionization of a π electron.
(c) The base peak in the EI mass spectrum of 1-heptene occurs at m/z = 41, which is believed to correspond to the allyl cation, a resonance-stabilized carbocation.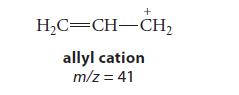
Draw a curved-arrow mechanism (or fishhook mechanism) that shows the conversion of the molecular ion you drew in part (b) into the allyl cation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





