(a) Give the product X expected when methylenecyclobutane undergoes acid-catalyzed hydration. (b) The rate-limiting step is protonation...
Question:
(a) Give the product X expected when methylenecyclobutane undergoes acid-catalyzed hydration.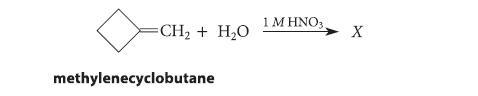
(b) The rate-limiting step is protonation of the double bond; use H3O+ as the acid catalyst. Draw the structure of the reactive intermediate formed in the rate-limiting step.
(c) Draw the transition state for the rate-limiting step.
(d) What is the rate-limiting step for dehydration of X (the reverse of the reaction shown above)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





