(a) Given the stretching frequencies for the CH bonds shown in color, arrange the corresponding bonds in...
Question:
(a) Given the stretching frequencies for the C—H bonds shown in color, arrange the corresponding bonds in order of increasing strength. Explain your reasoning.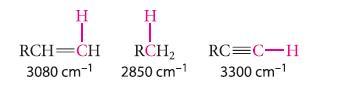
(b) If the bond dissociation energy of the = C—H bond is 558 kJ mol–1 (133 kcal mol–1), use the stretching frequencies in part (a) to estimate the bond dissociation energy of the C—H bond in RCH2—H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





