(a) Use the picture of the catalyst complex in Fig. 11.3a to explain why most E allylic...
Question:
(a) Use the picture of the catalyst complex in Fig. 11.3a to explain why most E allylic alcohols undergo asymmetric epoxidation more rapidly than their Z isomers.
(b) Would the same phenomenon be observed with (2)-DET, the enantiomer of the DET used in Fig. 11.3? Explain.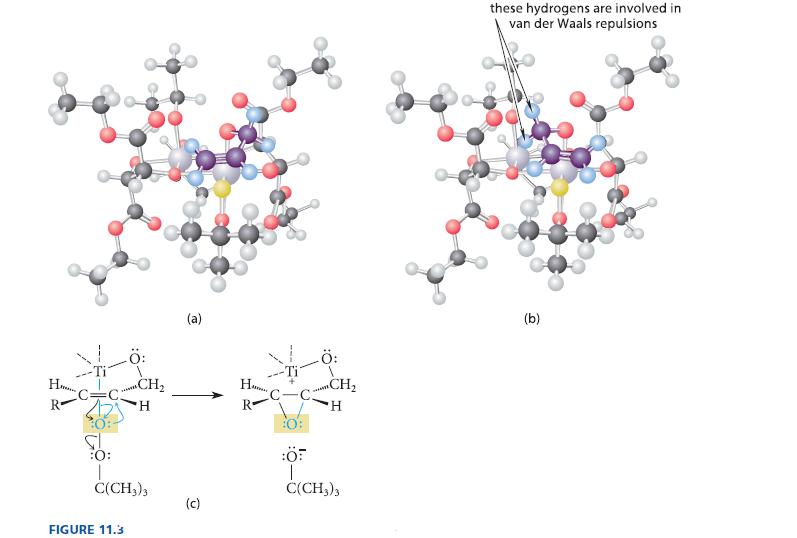
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





