Propose a synthesis for each of the following compounds in enantiomerically pure form. Use an asymmetric epoxidation
Question:
Propose a synthesis for each of the following compounds in enantiomerically pure form. Use an asymmetric epoxidation in each synthesis.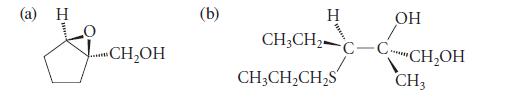
Transcribed Image Text:
(a) H ...CH₂OH (b) H CH3CH₂ CH3CH₂CH₂S C OH CH₂OH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
3 b H H CHCH DET TiOPr4 CH...View the full answer

Answered By

Chaudhary Deepa Tomar
I graduated with Bachelor of Science in 2015 from CCSU,Meerut. I have certificate in CCC computer language. Now I am pursuing my B.ed from CCSU,Meerut .This education helped further from my analytical mind.My analytical mind of thinking is well suited to teacher role where I oversee students
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline a synthesis for each of the following compounds in enantiomerically pure form from enantiomerically pure (2R,3R)-2,3-dimethyloxirane: (a) (b) (c) (d) (2R,3S)-3-methoxy-2-butanol O T ||...
-
Outline a synthesis for each of the following compounds in stereochemically pure form from enantiomerically pure (2R,3R)-2,3-dimethyloxirane: (a) (b) CH,O O (3S)-CH CHC CH ., , , OCH (2S,3R)-CH CH...
-
Propose a synthesis for each of the following compounds from butyric acid and any other reagents. (a) 2-methyl-2-pentanol (b) CH3CH2CH2CH2CH2CH2NH2 (c) CH3CH2CH2CH2NH2
-
If the Federal Government increases taxes:What will be the effect on money demand, money supply, and interest rates? Money demand decreases, money supply is unchanged, and interest rates decrease...
-
Kim Lee, the bookkeeper for Briton Company, had never missed a days work for the past 10 years until last week. Since that time, he has not been located. You now suspect that Kim may have embezzled...
-
A woman borrows K^(80000) to start a business. The bank charges 20% for the duration of the loan repayment period. How much would she pay per month if she is to repay the loan in two years?
-
Is the QMDM overly sensitive to changes in assumptions?
-
The American Produce Company purchased a truckload of cantaloupes (weighing 4,000 pounds) for $800. American Produce separated the cantaloupes into two grades: superior and economy. The...
-
6. Which of the following statements are correct with respect to the market value of equity (MVE)? There may be more than one correct answer to this question. a) MVE is equal to the sum between...
-
Give the major organic product of each of the following reactions. Include stereochemistry where relevant. (a) Dibutyl sulfide with 1 equivalent of H 2 O 2 at 25C (b) Dibutyl sulfide with 2 or more...
-
(a) Use the picture of the catalyst complex in Fig. 11.3a to explain why most E allylic alcohols undergo asymmetric epoxidation more rapidly than their Z isomers. (b) Would the same phenomenon be...
-
Ed's Lawn Service entered into the following transaction during 2012, its first year of operations: 1. Collected $12,000 in cash in cash from shareholders. 2. Borrowed $5,000 from a bank. 3....
-
1. (5 pts) Given y[n]= 2y[n-1] and y[0]=2, Write MATLAB code to calculate and plot y for 0
-
F ( t ) = t 4 + 1 8 t 2 + 8 1 2 , g ( t ) = ( t + 3 ) / 3 ; find ( f o g ) ( 9 )
-
How did they calculate allocated cost FLIGHT A FLIGHT 350 615 FLIGHT 3 1 Go GALS 20 G EXISTING SCHEME, DETERMINE THE OVE OR FLIGHTS A, B, AND C. 2 ED AT 7.00 PER K1.00 OF PILOT SALAF TOTAL NON-SALARY...
-
High Tech ManufacturingInc., incurred total indirect manufacturing labor costs of $540,000. The company is labor-intensive. Total labor hours during the period were 5,000. Using qualitativeanalysis,...
-
Start with AS/AD and IS/MP in full employment equilibrium. Assume the is a massive positive aggregate demand shock. How would this affect AS/AD and IS/MP and prices and output relative to the full...
-
Suppose that a continuous function is periodic with period 2 and is quadratic between -0.25 and 0.25 and linear between - 1.75 and - 0.25. In addition, it has the value 0 at 0 and 0.0625 at 0.25....
-
An 8.0 kg crate is pulled 5.0 m up a 30 incline by a rope angled 18 above the incline. The tension in the rope is 120 N, and the crates coefficient of kinetic friction on the incline is 0.25. a. How...
-
Proteins can be cleaved specifically at the amide bond on the carboxyl side of methionine residues by reaction with cyanogen bromide, BrC = N. The reaction occurs in several steps: (a) The first step...
-
A clever new method of peptide synthesis involves formation of an amide bond by reaction of an -keto acid with an N-alkyl hydroxylamine: The reaction is thought to occur by nucleophilic addition of...
-
Arginine, the most basic of the 20 common amino acids, contains a guanidino functional group in its side chain. Explain, using resonance structures to show how the protonated guanidino group...
-
Nitin is paid a base salary of $200 per week and commission at the rate of 3% for sales over $5000, 4% if his sales are over $8000, and 5% if sales are over $15,000. How much will Nitin earn in a...
-
Safa is paid a base salary of $1500 per month and a commission of 6% on all sales over $75,000. Last month, Safa's gross salary was $4440. What were her sales for the month? a$149,000 b$124,000...
-
Your regular hourly rate of pay is $15.86, and you are paid double time for all work on weekends and for any time over forty hours per week (Monday to Friday). Calculate your gross earnings for a...

Study smarter with the SolutionInn App


