Account with a mechanism for the fact that the hydrolysis of trimethyl phosphate to dimethyl phosphate in
Question:
Account with a mechanism for the fact that the hydrolysis of trimethyl phosphate to dimethyl phosphate in acidic solution containing 18O-labeled water gives methanol containing 18O and dimethyl phosphate containing no isotope, as shown in Fig. P25.30.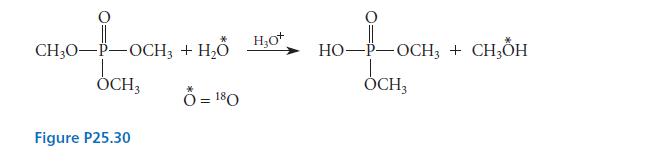
Transcribed Image Text:
40-100 CH₂0-P-OCH3 OCH3 Figure P25.30 + H₂O H₂O * Ô =180 H₂0+ 10-1-OCK HO-P-OCH3 + CH3OH OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
CH0P OCH3 HO ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The mechanism for acidic hydrolysis of a nitrile resembles the basic hydrolysis, except that the nitrile is first protonated, activating it toward attack by a weak nucleophile (water). Under acidic...
-
Suppose we have some optically pure (R)-2-butyl acetate that has been "labeled" with the heavy 18O isotope at one oxygen atom as shown. (a) Draw a mechanism for the hydrolysis of this compound under...
-
The pesticide Atrazine (C 8 H 14 C1N 5 , mol wt. 216 g/ mol) degrades in soil by a first-order reaction process. Consider the situation shown in the figure below, where there is a spill of solid...
-
Mick Stone disposed of the following assets during tax year 2020-21: (1) On 19 May 2020, Mick sold a freehold warehouse for 522,000. This warehouse was purchased on 6 August 2008 for 258,000, and was...
-
A 0.05394-kg sample of 144 Nd (atomic mass 143.91 u) emits an average of 2.36 particles each second. Find the decay constant in s 1 and the half-life in years.
-
Firms will increase production when planned investment is less than (actual) total investment. Is this statement true, false, or uncertain? Explain your answer.
-
Describe the concept of earned value. AppendixLO1
-
The following activities take place at Lohman CPAs during a recurring external audit of Reliance Corp. Classify the activities as value-added or non-value-added from the perspective of Reliance Corp....
-
I write to you concerning one Mr. Joseph Merton of Big City, CT. I regret to tell you that Mr. Merton is currently undergoing treatment for a case of lead poisoning, suspected intentional. He...
-
The reaction of water with metaphosphate ion is shown in Eq. 25.19b. Could nitrate ion undergo an analogous reaction with aqueous NaOH? If so, draw the structure of the product. If not, explain why....
-
Methanol containing the oxygen isotope 18 O is allowed to react, in separate reactions, with each of the acid chlorides shown in Fig. P25.29ac, and each of the resulting compounds A, C, and E is...
-
Sosa Corporation recently reported an EBITDA of $31.3 million and net income of $9.7 million. The company had $6.8 million in interest expense, and its average corporate tax rate was 35 percent. What...
-
Waverly Company Ltd. currently produces 8,000 units per year of SB 200 (snowboard), which is a component of the company's major products. SB 200 has the following unit cots Direct materials - $35.50...
-
Norton Ltd manufactures a single product, which is sold for $150 per unit. The standard variable costs per unit of the product are: Direct material 4 kilos at $8 per kilo Direct labour 5 hours at $10...
-
QUESTION 4 Murni Selasih Bhd is considering investing in a project that will generate higher returns Currently, the company has two projects with forecasted outcomes under consideration. The possible...
-
ABC plans to sell 60,000 units of product 751 in June, and each of these units requires five sq. ft. of raw material. Additional data is as follows: Product Raw No. 751 Material Actual June 1 11,200...
-
Case: Tom has felt anxious and constantly on edge over the past 3 years. He has few social contacts because of his nervous symptoms. He is married with 3 children and worries about if he is a good...
-
Why is a slow neutron more likely to induce a nuclear reaction (as in neutron activation and induced fission) than a proton with the same kinetic energy?
-
Match the following. Answers may be used more than once: Measurement Method A. Amortized cost B. Equity method C. Acquisition method and consolidation D. Fair value method Reporting Method 1. Less...
-
Identify each of the following compounds from their spectra. (a) Compound A: molecular mass 113; gives a positive hydroxamate test; IR 2237, 1733, 1200 cm-1; proton NMR: 1.33 (3H, t, J = 7 Hz), ...
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) 1-cyclohexyl-2-methyl-2-prupanol from bromocyclohexane (b) PhNHCH2CH2CH(CH3)2...
-
Rationalize each of the reactions in Fig. P21.56 with a mechanism, using the curved-arrow notation where possible. In part (d), identify compound A and show the mechanism for its formation. (Do not...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


