The reaction of water with metaphosphate ion is shown in Eq. 25.19b. Could nitrate ion undergo an
Question:
The reaction of water with metaphosphate ion is shown in Eq. 25.19b. Could nitrate ion undergo an analogous reaction with aqueous NaOH? If so, draw the structure of the product. If not, explain why.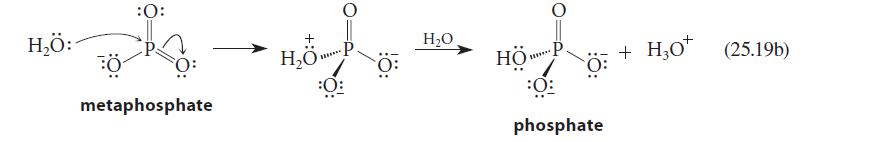
Transcribed Image Text:
H₂Ö: :O: metaphosphate H₂O P ***** :0 H₂O HÖP. ******* phosphate H30* (25.19b) + H₂0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Nitrate ion is stabilized by resonance which distributes the negative charge ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reaction of water with CH3Cl in acetone as a solvent is represented by the equation CH3Cl H2O CH3OH + HCl The rate of the reaction doubles when the concentration of CH3Cl is doubled and it...
-
Cocaine metabolism in rats can be studied by injecting the drug and periodically withdrawing blood to measure levels of metabolites by HPLC-mass spectrometry. For quantitative analysis, isotopically...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
In the year to 5 April 2021, Thomas More made the following disposals: (i) A flat in a house that he had purchased on 1 December 2010 for 80,000. It had never been occupied as the main residence and...
-
The isotope 24 Na is a emitter with a half-life of 15 h. A saline solution containing this radioactive isotope with an activity of 600 kBq is injected into the bloodstream of a patient. Ten hours...
-
If the Bank of Canada has an interest-rate target, why will an increase in the demand for reserves lead to a rise in the money supply? Use a graph of the market for reserves to explain.
-
What is a burn-down chart? What purpose does it serve? AppendixLO1
-
Journalize the adjusting entry needed on December 31, end of the current accounting period, for each of the following independent cases affecting Green Corp. Include an explanation for each entry. a....
-
Clear my choice Which of the following is not a method for calculating GDP? Select one: O Total domestic tax revenue. O Total domestic income. O Total spending on domestic final products. O Total...
-
The conjugation of alcohols containing nonpolar groups (for example, phenol) to glucuronic acids is a key process by which these alcohols can be transformed into, and excreted as, water-soluble...
-
Account with a mechanism for the fact that the hydrolysis of trimethyl phosphate to dimethyl phosphate in acidic solution containing 18 O-labeled water gives methanol containing 18 O and dimethyl...
-
Based on the population growth analysis, a citys wastewater is estimated to increase over time, as depicted in Figure 9.23 . Four phases for treatment plant expansion capacity are studied for the...
-
Given below is some is a comparison of financial performance data of a project when flexibility is incorporated (I.e. flexible project) in comparison to when it is not. (i.e. inflexible project) The...
-
For Service Zone H, assuming your shipment chargeable weight is between 100 and 300 kg, at what weight does it become cheaper to declare the shipment weight to be 300 kg.? EG: What is the rate break...
-
Gold Dust Ltd has produced the following budgeted data for its current financial year:- Sales 2900000 Direct materials 400000 Direct labour 500000 Production overhead 1200000 Production cost 2100000...
-
Critical Review V Hide Assignment Information Instructions Williams, A. (2012). Worry, intolerance of uncertainty, and statistics anxiety. Click on the following link to retrieve the article....
-
(4.) Octopussy Company uses a predetermined overhead rate in applying overhead to production orders on a labor-cost basis for Dept. A and on a machine-hour basis for Dept. B. At the beginning of...
-
Why can we ignore the binding energies of the atomic electrons in calculations such as Example 29.4? Isn't there a mass defect due to the binding energy of the electrons?
-
Using thermodynamic data from Appendix 4, calculate G at 258C for the process: 2SO 2 (g) + O 2 (g) 88n 2SO 3 (g) where all gases are at 1.00 atm pressure. Also calculate DG8 at 258C for this same...
-
Pentanoic acid and methyl butyrate are constitutional isomers. Which has the higher boiling point and why?
-
Klutz McFingers. a graduate student in his ninth year of study, has suggested the following synthetic procedures and has come to you in the hope that you can explain why none of them works very well...
-
Complete the reactions given in Fig. P21.52 by giving the principal organic products. Explain how you arrived at your answers. NaOH CH O (trace) H,C CCHO CH +CH OH (solvent) Ph NH2 1 (CgH,NO3)...
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


