An amino acid A, isolated from the acid-catalyzed hydrolysis of a peptide antibiotic, gave a positive test
Question:
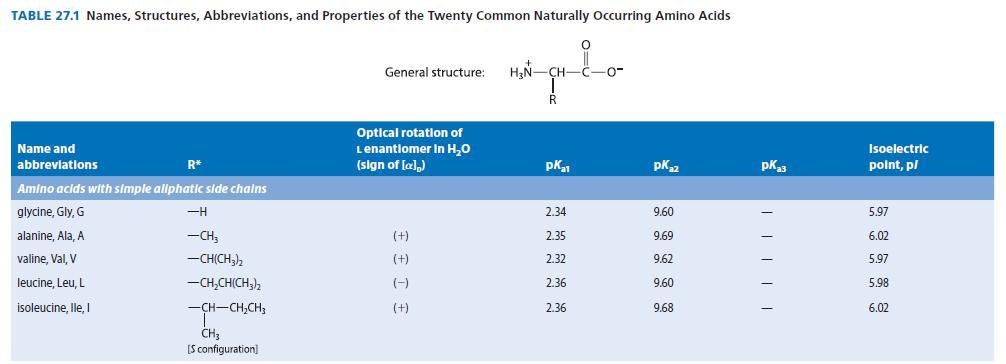
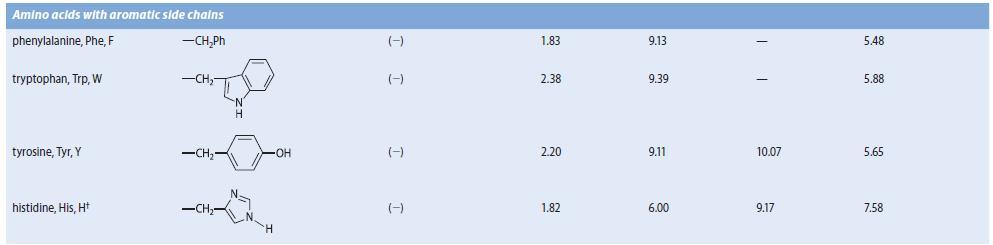
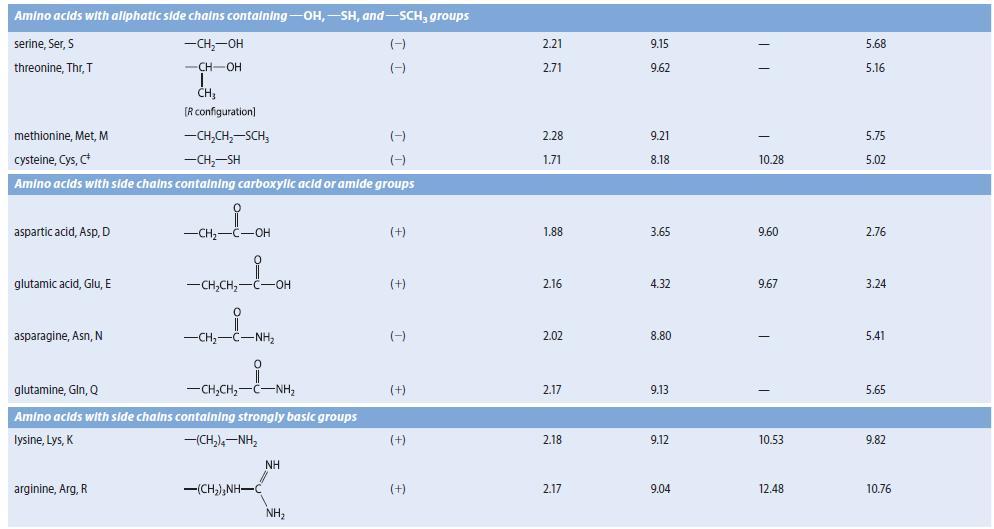
An amino acid A, isolated from the acid-catalyzed hydrolysis of a peptide antibiotic, gave a positive test with ninhydrin and had a specific optical rotation (HCl solution) of 137.5° mL g–1 dm–1. Compound A was not identical to any of the amino acids in Table 27.1. The isoelectric point of compound A was found to be 9.4. Compound A could be prepared by the reaction of l- glutamine with Br2 in NaOH, followed by neutralization. (See Sec. 23.11D.) Suggest a structure for A.
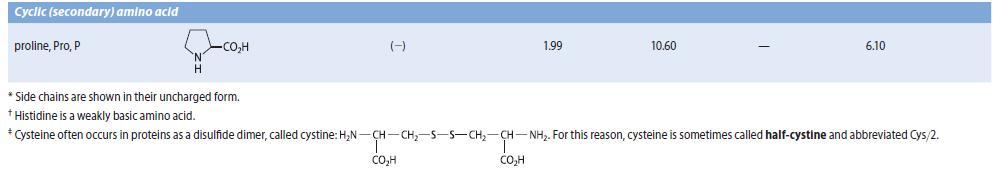
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





