Arrange the following four bases in descending order with respect to the E2 elimination to S N
Question:
Arrange the following four bases in descending order with respect to the E2 elimination to SN2 substitution product ratio expected when they react with isobutyl bromide. Explain your answers.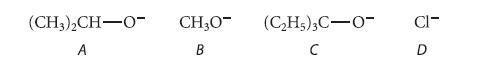
Transcribed Image Text:
(CH3)2CH-O- A CH₂O B (C₂H5)3C-O- C CI- D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Chloride ion is a weaker base tha...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the following four alkyl halides in descending order with respect to the E2 elimination to S N 2 substitution product ratio expected in their reactions with sodium ethoxide in ethyl alcohol....
-
The following reaction is a common synthesis used in the organic chemistry laboratory course. When we double the concentration of methoxide ion (CH3O-), we find that the reaction rate doubles. When...
-
Discuss the purposes of PE in relation to one's everyday activities. To develop optimum physical fitness and health of the individual so that he is capable of living the "good life" and contributing...
-
Write structures for the following bicyclic alkanes: (a) Bicyclo [1.1.0] butane (b) Bicyclo [2.1.0] pentane (c) 2-Chlorobicyclo [3.2.0] heptane (d) 7-Methylbicyclo [2.2.1] heptane
-
The Town of Quincys fiscal year ends on June 30. The following data relate to the property tax levy for the fiscal year ended June 30, 2012. Prepare journal entries for each of the dates as...
-
For nongovernmental NFP, how are unconditional promises to give with collections due in the next period accounted for?
-
P 16-12 Partnership income allocation The partnership of Par and Boo was formed and commenced operations on March 1, 2016, with Par contributing $30,000 cash and Boo investing cash of $10,000 and...
-
A quarterback throws a football straight toward a receiver with an initial speed of 20.0 m/s, at an angle of 30.0 above the horizontal. At that instant, the receiver is 20.0 m from the quarterback....
-
For calendar 2014, Gomez Corporation reported pre-tax income of $70,000. A recount of the company's inventory revealed that 2014 ending inventory was overstated by $10,000. What is Gomez's corrected...
-
Write a curved-arrow mechanism for formation of the rearrangement product shown in Eq. 9.60. CH3 CH3 I T H3C-C- CH 3 -CH-Cl EtOH 80 C CH3 CH3 | I H3C-C- | OEt + -CH-CH3 EtOH Cl + other products...
-
What nucleophile or base and what type of solvent could be used for the conversion of isobutyl bromide into each of the following compounds? (a) (CH3)CHCHS (CH3)2 Br (b) (CH3)CHCHSCHCH3 (c)...
-
Plastic Products is concerned about the inside diameter of the plastic PVC pipe it produces. A machine extrudes the pipe, which is then cut into three-metre lengths. About 720 pipes are produced per...
-
As shown on the attached chart, what is the approximate current 7-year spread premium for Kellogg Bonds? 25 Basis Points 75 Basis Points 200 Basis Points AUS Treasury Actives Curve X-ads Tenor...
-
A pharmaceutical company claims to have invented a new pill to aid weight loss. They claim that people taking these pills will lose more weight than people not taking them. A total of twenty people...
-
Let U = {a, b, c, d, e, f} be the universal set and let A = {a, b, c, d, e, f}. Write the set A. Remember to use correct set notation. Provide your answer below: A=
-
Produce a poster series of three (3) A3 sized posters on creativity in the early years. As a collective the poster series must articulate the importance of aesthetics and creativity for young...
-
Find the second derivative of the function. g(x) = ex In(x) g"(x) = Need Help? Read It
-
Suppose that one has an unlimited supply of identical blocks each 1 unit long. (a) Show that they may be stacked as in Figure 8 without toppling. Consider centers of mass. (b) How far can one make...
-
Gopher, Inc. developing its upcoming budgeted Costs of Quality (COQ) with the following information: Expense Item Budget Raw Materials Inspection $ 15,000 EPA Fine 200,000 Design Engineering 15,000...
-
Terminal alkynes react with Br2 and water to yield bromo ketones. For example: Propose a mechanism for the reaction. To what reaction of alkenes is the processanalogous? -CECH Br2, H20 CH2Br
-
A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene. What kind of hybridization do the two central carbon atoms have? What is the geometric relationship...
-
Reaction of acetone with D 3 O + yields hexadeuterioacetone. That is, all the hydrogen?s in acetone are exchanged for deuterium. Review the mechanism of mercuric ion?catalyzed alkyne hydration, arid...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


