Classify the isomeric carbocations in each of the following parts as primary, secondary, or tertiary, and tell
Question:
Classify the isomeric carbocations in each of the following parts as primary, secondary, or tertiary, and tell which is the most stable carbocation in each part and why.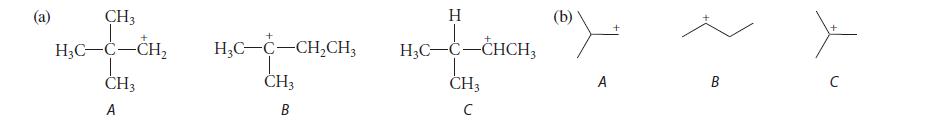
Transcribed Image Text:
(a) CH3 H₂C-C-CH₂ I CH3 A + + HỌC–C–CH,CH, CH3 B H H3C-C-CHCH3 CH3 с (b) A B с
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a CH3 CCH CH3 HCC a primary carbocation least stable b a ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
question is based on KMP algorithm and string matching algorithm: b) (5 marks) Given two strings, A = a1, a2,...am and B = b1, b2, ...bn, we define a matrix C[0..m, 0..n] as follows: (1 i m, 1 j n)...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
11. Assume that the total cost function (TC) is given by the following equation: TC = 100+ 2.5y + 0.05 y2, where y is output. a. What is the total fixed cost (TFC)? b. What is the average total cost...
-
How did the court rule on the prospective advantage claim, and why?
-
(a) Based on the lattice energies of MgCl2 and SrCl2 given in Table 8.2, what is the range of values that you would expect for the lattice energy of CaCl2? (b) Using data from Appendix C, Figure 7.9,...
-
A security code consists of three letters followed by one digit. The first letter cannot be an A, B, or C. What is the probability of guessing the security code in one trial?
-
1. Investment is a larger component of GDP than consumption, but it is much more volatile. _____ (True/False) 2. Investment spending is very_______, since it moves in conjunction with GDP. 3. The...
-
Cork Bottlers has 84 million shares outstanding and expects earnings at the end of this year of $54 million. Cork plans to pay out 30% of its earnings as a dividend and 10% of its earnings through...
-
Using the known regioselectivity of hydrogen halide addition to alkenes, predict the addition product that results from the reaction of: (a) HCl with 2-methylpropene (b) HBr with 1-methylcyclohexene
-
Give the structure of the addition product formed when ethylene reacts with each of the following reagents: (a) HI (b) Br 2 (c) BH 3 Each of the BH bonds undergoes an addition to one molecule of...
-
Consider a risk-neutral firm, protected by limited liability, that wants to finance a project at a cost \(I=1\). The project takes one period to complete. The firm has no initial wealth; hence to...
-
What should be the equivalent units of production for (1) Dept M and (2) Dept. P? Can you please show the solutions and answer. Thanks Problem 1 Lee Gon Mfg. Co has its product processed in two...
-
Moullierat Mfg. is considering a rights offer. The company has determined that the ex-rights price will be $95. The current price is $102 per share, and there are 24 million shares outstanding. The...
-
This question involves hypothesis testing. The following numbers will help you answer these questions. The random variable Z ~N(0, 1) is standard normal. P(Z >1.28).1 P(Z1.65) .05 P(Z1.96) .025 P(Z...
-
Human service organizations require strong and effective leadership. Understanding what qualities make up an effective leader and how these qualities can be cultivated is of critical importance for...
-
18. What is the name of the heat treatment performed on a cold worked sample? 19. What is the percent coldwork of a sample with an initial thickness of 11mm and a final thickness of 7mm? 20. Which...
-
Michael P. was very weak from birth, with poor muscle tone, difficulty breathing, and great fatigue. By his third month, he began having seizures. Michael's medical tests were normal except for ore:...
-
Cleaning Service Company's Trial Balance on December 31, 2020 is as follows: Account name Debit Credit Cash 700 Supplies Pre-paid insurance Pre-paid office rent Equipment Accumulated depreciation -...
-
Draw structures of the nine isomers of C7H16.
-
In each of the following sets, which structures represent the same compounds and which represent different compounds? CH Br C (a) " , n CHH CH3CHCHCH3 Br Br CH . (b) , " (c) CH CH2CH3 CH H,H,, ,...
-
There are seven constitutional isomers with the formula C4H10O. Draw as many as you can.
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


