Consider the reaction of a methyl radical (CH3) with the p bond of an alkene: The relative
Question:
Consider the reaction of a methyl radical (·CH3) with the p bond of an alkene: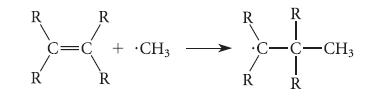
The relative rates of the reaction shown in Fig. P5.50 were determined for various alkenes.
(a) Draw the free-radical product of the reaction in each case and explain.
(b) Explain the order of the relative rates.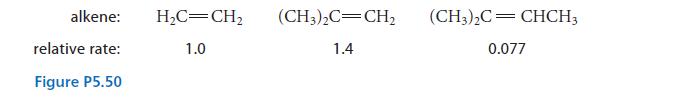
Transcribed Image Text:
R R T Hoa C=C + CH3 R R R R R R •C-C-CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a The methyl radical reacts at the carbon of the double bond with fewer substituents because this mode of reaction gives the more substituted radical ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reaction of a methyl ketone with iodine solution in the presence of aqueous sodium hydroxide is shown in the equation below: RCOCH3() + 3l2(aq) + 4NaOH(aq) RCOONa(aq) + CHl3(s) + 3Nal(aq) +...
-
Namib Mills, has a fixed total capital of $10,000,000, which is made up of 20 percent debt and 80 percent equity. The firm has 100,000 outstanding ordinary shares and no preference shares. Although...
-
Suppose an industry has the following data: TC=q-5q+100 a P=55-2Q If the industry is competitive; Calculate price, production quantity and profit. b. If there is a firm in the industry; Calculate...
-
Think about the impact that e-commerce and more flexible networks of organisations has had on the way international business is conducted. How has it created new complexities in the relationships...
-
H. Banks Company would like to design, produce, and sell versatile toasters for the home kitchen market. The toaster will have four slots that adjust in thickness to accommodate both slim slices of...
-
Write SQL retrieval commands for each of the following queries: a. Display the course ID and course name for all courses with an ISM prefix. b. Display all courses for which Professor Berndt has been...
-
Will we have a contract? All verbal promises should be in writing before you pay any money.
-
Nancy Nanny opened a child-care facility on January 1, Year 1. Use the Chart of Accounts below to complete the requirements on the following pages for Nancy Nanny Child Care. NANCY NANNY CHILD...
-
During April, the first production department of a process manufacturing system completed its work on 355,000 units of a product and transferred them to the next department. Of these transferred...
-
Equations 5.25ac on p. 193 show the formation of trialkylboranes from alkenes and BH 3 . In the reaction of 2,3-dimethyl-2-butene with BH 3 , only two equivalents of the alkene react, even with a...
-
Using the curved-arrow or fishhook notation, as appropriate, suggest mechanisms for each of the reactions given in Fig. P5.49. (a) HC (b) H3C C-CH CHCH=CH HC CH T T HO OH C#yan + Br H3O+ HC + CI-S-CI...
-
For Exercises perform each of the following steps. a. State the hypotheses and identify the claim. b. Find the critical value(s). c. Find the test value. d. Make the decision. e. Summarize the...
-
The pipe assembly is mounted vertically as shown in (Figure 1). Part A Figure 200 mm 80 mm- 1.2 m Determine the pressure at A if the velocity of the water ejected from B is 0.77 m/s. Express your...
-
Suppose you were asked to estimate the probability that your coin came up "Heads" from your observations. Provide such an estimate including a 90% confidence interval. Explain your calculations.
-
Given the following C++ code snippet, what is the value of num3? Your answer must be exact. int num1, num2, num3; int *p_num1 - &num1; int *p_num2 &num2; *p_num1 15; *p_num2 10; * num3 = *p_num1...
-
# 1. On April 1, C.S. Lewis Company borrows (Notes payable) $70,000 from Lyon National Bank by signing a 3 month 8% note. Prepare the journal entries to record: a) the issuance of the note b) the...
-
Find and correct the program below, check your answer by running the script 1. 2. #include int main() 3. { 4. float Number1; 5. double Number2; 6. 7. 8. printf("Enter a number: "); scanf("%f",&num1)...
-
Explain which the independent variable is and which the dependent variable is for each of the following examples. a. Once you determine the price of a notebook at the college bookstore, you will...
-
6 (a) Briefly develop a mathematical model of the behaviour of a copper-twisted pair cable (b) Derive the magnetic energy from: w given that: K + w, where the - - k symbols have their usual meaning...
-
The energy level diagram for the MOs of CH 2 = CH 2 is shown if Figure 3.24. Show a similar diagram for the lowest-energy excited state of this molecule. C=C 5 o* * Ethene 5 o Energy
-
Consider the species formed by the addition of an extra electron to H2 so that there are three electrons and a negative charge. Show an energy level diagram for the MOs of this species. Is there...
-
Draw an energy level diagram for the excited state of H2. Is there still a bond between the hydrogens?
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


