Decide whether each of the following compounds is aromatic. Explain your reasoning. (a) (d) -CH3 toluene (b)
Question:
Decide whether each of the following compounds is aromatic. Explain your reasoning.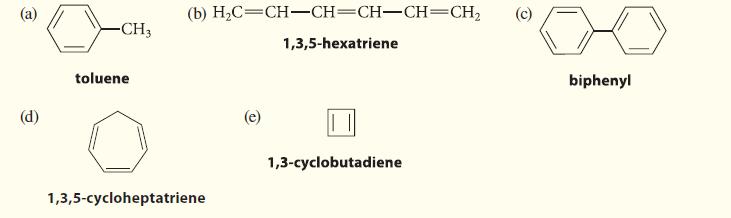
Transcribed Image Text:
(a) (d) -CH3 toluene (b) H₂C=CH-CH=CH-CH=CH₂ 1,3,5-hexatriene 1,3,5-cycloheptatriene (e) 1,3-cyclobutadiene (c) biphenyl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
In each example first count the electrons by applying the following rule Each double bond contribute...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Each of the following compounds is an aromatic compound bearing a substituent that we did not discuss in this chapter. Using the principles that we discussed in this chapter, predict the major...
-
Determine whether each of the following compounds is a cis isomer or a trans isomer a. b. c. d. e. f. Cl Br CH3 CH3 Br Br CH3 Cl CH3 CH3 CH
-
Predict whether each of the following compounds is likely to be dangerously explosive in contact with BrF 3 and explain your answer: (a) SbF 5 , (b) CH 3 OH, (c) F 2 , (d) S 2 Cl 2 .
-
discuss the benefits and challenges of the employee job performance evaluation process as it relates to the ratings of an individual. For example, if an employer uses a standard Likert Scale (1...
-
Give an example of an asset exchange transaction. What is the effect of this transaction on the accounting equation?
-
A local supermarket manager wants to use two independent variables, customer age (in years) and whether the customer subscribes to the supermarket chain's health/wellness e-newsletters (coded as 1 =...
-
Take T = 2. Suppose consumption C0 is known at date 0 (before any coins are tossed). Assume the power certainty equivalent and the CES aggregator. (a) Assume two coins are tossed at date 0...
-
Nonfinancial measures of quality, manufacturing cycle efficiency. (CMA, adapted) Torrance Manufacturing evaluates the performance of its production managers based on a variety of factors, including...
-
Posters com is a small Internet ietailer of high - quality posters. The compary has $ 7 0 , 0 0 0 in operatirig assets and fixed expenses of $ 1 5 3 . 0 0 0 per year. With this level of operating...
-
Explain why there is a larger difference between the heats of formation of (E)-1,3 - pentadiene and 1,4-pentadiene (29.3 kJ mol 1 or 7.1 kcal mol 1 ) than between (E)-1,3- hexadiene and...
-
Use the Frost circle to determine the energy levels and electron occupancies for the MOs of benzene.
-
What is the difference between the general purposes of informing, persuading, and building goodwill in oral communication? Provide one example for each general purpose.
-
Find the explained variation for the paired data. The equation of the regression line for the paired data below is y = 5.18286 + 3.33937x. X 972 23 34 4 22 17 y 43 35 16 21 23 102 81
-
5. The vertical stress at a point is 28 kPa, while the horizontal stress is 14 kPa. shear stress on the horizontal plane is +4 kPa. The (a) Draw the Mohr's circle of stress and show the pole point...
-
I need assistance with the below questions for my HIM 5370 at texas State University Case Mix Table: 4. Complete the Case Mix table shown below. Calculate the case mix for each month. The table below...
-
The probability that a printing press will print a book with no errors is 78%. The company is about to process an order of 30 books. Round decimals to 3 places or percentages to 1 decimal place. 9....
-
alculate Product Costs, using JOB COSTING SYSTEM. Please SHOW CALCULATION. Dream Chocolate Company: Choosing a Costing System TABLE 1 Typical Prices and Costs of Chocolate 641 1.25 oz. Bar 3.0 oz....
-
In Problem 1-3, assume that the function given are differentiable, and find the indicated derivative. 1. f'(t) if f(t) = h(g(t)) + g2(t) 2. G"(x) if G(x) = F(r(x) + s(x)) + s(x) 3. If f(x) = Q...
-
Why is homeostasis defined as the "relative constancy of the internal environments? Does negative feedback or positive feedback tend to promote homeostasis?
-
Propose a structure for compound C, which has M + = 86 in its mass spectrum, an IR absorption at 3400 cm 1 , and the following 13 C NMR spectral data: Compound C Broadband-decoupled 13 C NMR: 30.2,...
-
Compound D is isomeric with compound C (Problem 13.61) and has the following 13C NMR spectral data. Propose a structure. Compound D Broadband-decoupled 13C NMR: 9.7, 29.9, 74.4, 114.4, 141.4 DEPT-90:...
-
Propose a structure for compound E, C7H12O2, which has the following 13C NMR spectral data: Compound E Broadband-decoupled 13C NMR: 19.1, 28.0, 70.5, 129.0, 129.8, 165.8 DEPT-90: 28.0, 129.8 ...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


