Determine the isomeric relationship between the following two molecules: CH3CH H CHCH3 H H C=C CH3CH CHCH3
Question:
Determine the isomeric relationship between the following two molecules: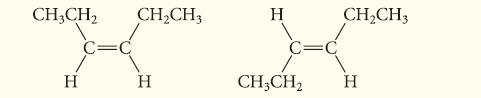
Transcribed Image Text:
CH3CH₂ H CH₂CH3 H H C=C CH3CH₂ CH₂CH3 H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Work from the top of Fig 611 and answer each question in turn These two molecules ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The two isomeric compounds below are naturally occurring insect pheromones. The isomer on the left attracts the male olive fruit fl y; the one on the right, the female. (a) What kind of isomeric...
-
Get-the-lead-out manufactures generic #2 pencils. Its cost function is C(q) 1250 +2q, where q is the number of boxes of pencils it produces each quarter (throughout, quantities and prices refer to...
-
Your team is faced with a puzzle. Four isomeric compounds, A D, with the molecular formula C 4 H 9 BrO react with KOH to produce E G with the molecular formula C 4 H 8 O. Molecules A and B yield...
-
0 The initial substitution of x = a yields the form Simplify the function algebraically, or use a table or 0 graph to determine the limit. When necessary, state "DNE". x + 4x-5 x - 1 Step 1: Factor...
-
Why did the judge direct a verdict in favor of Armani?
-
The following diagram represents a high-temperature reaction between CH4 and H2O. Based on this reaction, how many moles of each product can be obtained starting with 4.0 mol CH4?
-
What is the probability that a randomly selected firm will have at least 10 employees?
-
Rebecca S. Dukat arrived at Mockingbird Lanes, a bowling alley in Omaha, Nebraska, at approximately 6:00 P.M. to bowl in her league game. The bowling alleys parking lot and adjacent sidewalk were...
-
Assume the following information for a capital budgeting proposal with a five-year time horizon $ 375,000 Initial investment: Cost of equipment (zero salvage value) Annual revenues and costs: Sales...
-
Show the planes and centers of symmetry (if any) in each of the following achiral objects. (a) The methane molecule (b) A cone (c) The ethylene molecule (d) The trans-2-butene molecule (e) The...
-
A sample of (S)-2-butanol has an observed rotation of 12.18 at 20 C. The measurement was made with a 2.0 M solution of (S)-2-butanol in methanol solvent in a sample container that is 10 cm long. What...
-
What criteria does the NLRB consider when determining whether an appropriate unit of employees has substantial mutuality of interests?
-
Contract for construction crew and equipment 8 Build parking lots Exterior lighting 11 7 20 12 Build foundation Start Interior Interior 12 9 electrical Final wiring finish Purchase 8 14 12 material...
-
Mad Hatter Enterprises purchased new equipment for $369,000, terms f.o.b. shipping point. Other costs connected with the purchase were as follows: State sales tax Freight costs Insurance while in...
-
Write down a C program that takes runs scored by a batsman and prints the status according to the following policy: Runs scored >80 50-79 30-49 10-29 <10 Grade Excellent 4 Very Good Good Average Poor
-
Consider the standard two-period maximization problem for investor j over s states of nature: Subject to S max u(c) + (s)u(c;}(s)) S=1 Cjo + q(s) C; (s) = Wjo +244) S=1 where all terms are as defined...
-
At what point should a leader cease gathering data, take the risk, and simply make the decision? Support your position.
-
Explain the functions of the subcutaneous layer.
-
For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g) 2F (g) Ke= 4.9 x 10-21 c) C (s) + O2 (g) d) H2 (g) + C2H4 (g) C2H6 (g) Ke =...
-
Tert-Butyl ethers can be prepared by the reaction of an alcohol with 2-methyipropene in the presence of an acid catalyst. Propose a mechanism for this reaction.
-
Meerwein?s reagent, triethyloxonium tetra-fluoroborate, is a powerful ethylating agent that converts alcohols into ethyl ethers at neutral pH. Show the reaction of Meerwein?s reagent with...
-
Safrole, a substance isolated from oil of sassafras, is used as a perfumery agent. Propose a synthesis of safrole from catechol (1,2-benzenediol). CH2CH=CH2 Safrole
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


