Draw the structure of the major form of each of the following compounds present in an aqueous
Question:
Draw the structure of the major form of each of the following compounds present in an aqueous solution containing initially one molar equivalent of 1 M HCl. Explain your reasoning.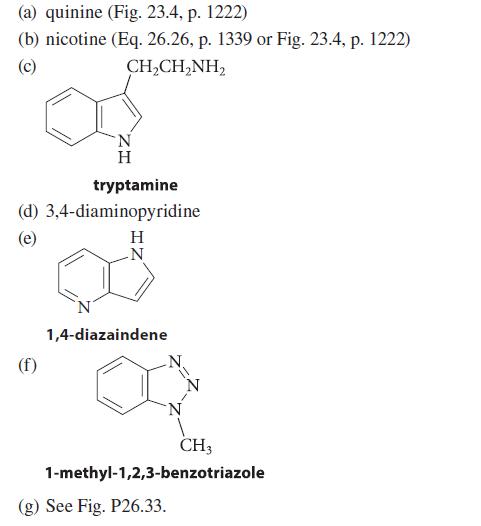
Transcribed Image Text:
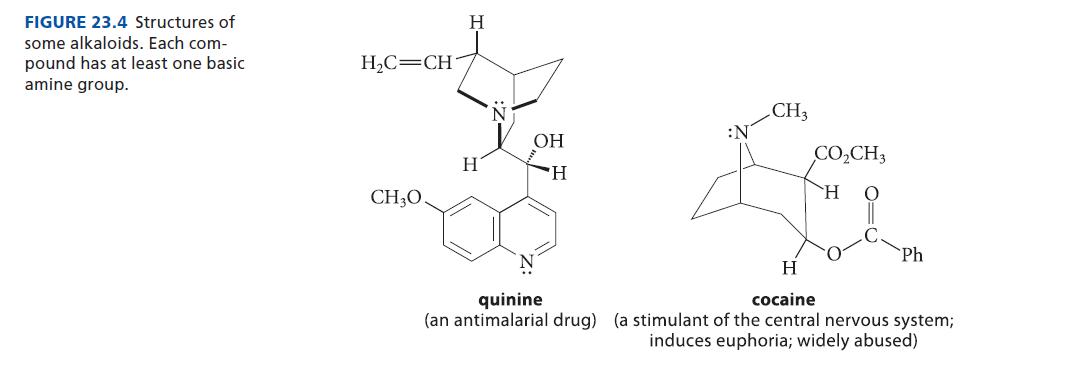
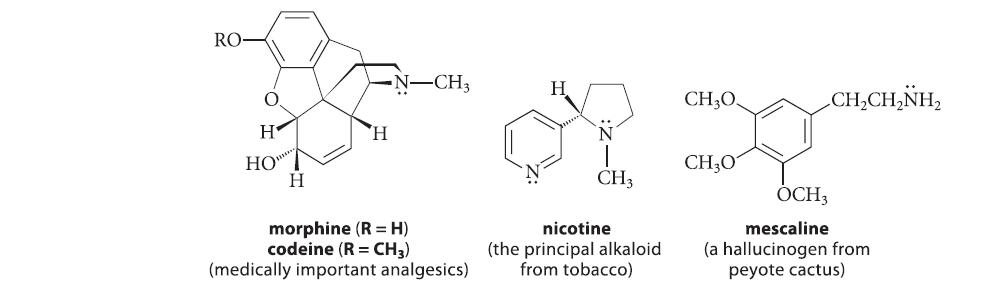
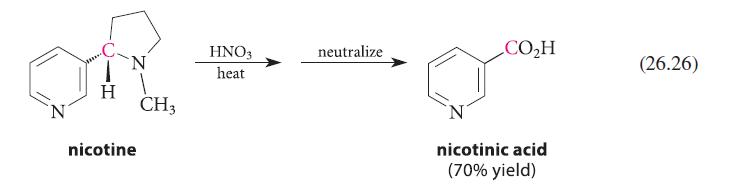
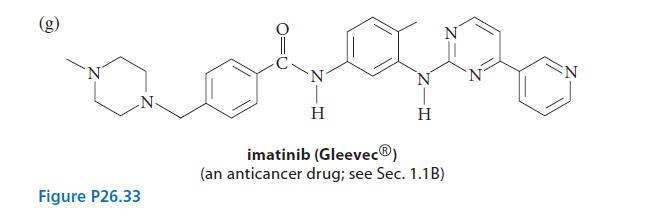
(a) quinine (Fig. 23.4, p. 1222) (b) nicotine (Eq. 26.26, p. 1339 or Fig. 23.4, p. 1222) (c) CH,CH,NH, (d) (e) H tryptamine 3,4-diaminopyridine H N 1,4-diazaindene N (g) See Fig. P26.33. CH3 1-methyl-1,2,3-benzotriazole
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a b Because alkylamines are more basic than pyridines or quinolines the conjugate acid of quinine is ...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What are the reasons that electric car drivers lease their cars If you were going to acquire an electric or hybrid vehicle, would you lease or purchase Explain your reasoning
-
An aqueous solution containing MgCl2 and HCl was analyzed by first titrating a 25.00-mL aliquot to a bromocresol green end point with 17.53 mL of 0.02932 M NaOH. A 10.00-mL aliquot was then diluted...
-
In an aqueous solution containing sodium bicarbonate, aniline reacts quickly with bromine to give 2, 4, 6-tribromoaniline. Nitration of aniline requires very strong conditions, however, and the...
-
Goods 1 and 2 are available at dollar prices of p1 per unit of Good 1 and p2 per unit of Good 2. A utility function U(x 1 , x 2 ) is a function representing the utility or benefit of consuming xj...
-
Rachael Ray Corporation had the following transactions. 1. Sold land (cost $12,000) for $15,000 2. Issued common stock for $20,000 3. Recorded depreciation of $17,000 4. Paid salaries of $9,000 5....
-
In a gasliquid interface mass-transfer process, the bulk mole fraction composition of solute A in the inert carrier gas is 0.010, and the bulk mole fraction composition of solute A in the inert...
-
Set the minimum antecedent support to 1%, the minimum rule confidence to 5%, and the maximum number of antecedents to 1. a. Generate rules using confidence difference as your evaluation measure with...
-
Polk Incorporated issued $200,000 of 13% bonds on July 1, 2013, for $206,801.60. The bonds were dated January 1, 2013, pay interest on each June 30 and December 31, are due December 31, 2017, and...
-
Cash Flows from (Used for) Operating Activities Indicate whether each of the following would be added to or deducted from net income in determining net cash flows from operating activities by the...
-
The following compound is a very strong base; its conjugate acid has a pK a of about 13.5. Give the structure of its conjugate acid and show that it is stabilized by resonance. CH3 -Z:
-
(a) Carry out an orbital symmetry analysis to show that suprafacial [1,5] carbon migrations should occur with retention of configuration in the migrating group. (b) Indicate what type of sigmatropic...
-
What five factors determine the strength of reference group influence in a situation? Discuss.
-
Using the figure below, draw the FBD , ?Shear Force and Bending Moment diagrams and find the maximum internal moment for the beam shown. 10 kNm 10 kN 3 m
-
Suppose the goods market is: Y = 1800 - 100i and the LM curve Y = 500 +591, where x is the last digit of your ID number. Determine the equilibrium income (Y), interest rate (i). Explain the role of...
-
Complete the chart: [1] Length, L (m) Period, T (s) LogL LogT 0.10 0.63 0.20 0.90 0.30 1.00 0.40 1.27 0.50 1.42 0.60 1.55 0.70 1.68 0.80 1.80 0.90 1.90 1.00 2.02 Plot the data T vs L. [4 (title, axes...
-
A solid metal sphere with a diameter of 2 cm and a mass of 8 g is used for the following heat transfer experiments. You can assume the temperature throughout the inside of the metal sphere is...
-
2) A car 1200 kg is in a skid Force of friction 3200N and force of air resistance 1600N. Find the following i) The net force and acceleration of the car ii) The time required to stop from 95 km/hr...
-
Why is the combination of direct labor and manufacturing overhead referred to as a conversion cost?
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
In each compound, identify (1) the diastereotopic fluorines, (2) the enantiotopic fluorines, (3) the homotopic fluorines, and (4) the constitutionally nonequirnalent fluorines. CH,
-
(a) When propanol containing deuterium (D, or 2H; rather than hydrogen at the oxyger, CH3CH2CH2OD, is treated with an excess of H2O containing a catalytic amount of NaOH, 1-propanol is formed...
-
Indicate whether each of the following transformations is an oxidation, a reduction, or neither, and how many electrons are involved in each oxidation or reduction process. (a) (b) OCHs + CH OH CH3...
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


