The following compound is a very strong base; its conjugate acid has a pK a of about
Question:
The following compound is a very strong base; its conjugate acid has a pKa of about 13.5. Give the structure of its conjugate acid and show that it is stabilized by resonance.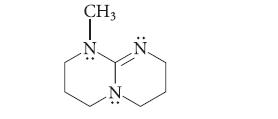
Transcribed Image Text:
CH3 -Z:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The conjugate acid is formed by prot...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What segment of the external environment has more impact on an organization? 2. If an organization wants to engage in a new industry, which area of the industry environment should be the most...
-
The following compound is a suicide inhibitor of the enzyme that catalyzes amino acid racemization: Propose a mechanism that explains how this compound irreversibly inactivates the enzyme. HC CCHO NH
-
Three bottles A, B. and C have been found, each of which contains a liquid and is labeled "amineC8H11N." As an expert in amine chemistry, you have been hired as a consultant and asked to identify...
-
Consider the utility function U(x 1 , x 2 ) = x 1 x 2 with budget constraint p 1 x 1 + p 2 x 2 = c. (a) Show that the maximum of U(x 1 , x 2 ) subject to the budget constraint is equal to c 2 /(4p 1...
-
Explain how organizations in the not-for-profit sector differ from organizations in the public sector or for-profit business sector. Provide an example of an entity in each sector.
-
A gasliquid convective mass-transfer process involves the transfer of the industrial contaminant methylene chloride (solute A) between air and water at 20C and 2.20 atm total system pressure. Air is...
-
Set the minimum antecedent support to 1%, the minimum rule confidence to 5%, and the maximum number of antecedents to 1. Use rule confidence as your evaluation measure. a. Find the association rule...
-
Molly Mocha employs one college student every summer in her coffee shop. The student works the five weekdays and is paid on the following Monday. (For example, a student who works Monday through...
-
Andretti Company has a single product called a Dak. The company normally produces and sells 85,000 Daks each year at a selling price of $58 per unit. The companys unit costs at this level of activity...
-
Which should be more reactive in nitration: -picoline or -picoline? Explain using resonance structures, and give the major nitration product(s) in each case.
-
Draw the structure of the major form of each of the following compounds present in an aqueous solution containing initially one molar equivalent of 1 M HCl. Explain your reasoning. (a) quinine (Fig....
-
Do you think more companies should establish wellness programs? Why or why not?
-
Sravani Bought an audio CD containing 1 0 0 audio files , 1 0 of which are by S . P . Balasubrahmanyam . Assume the shuffle option is enabled to play the songs in random order. What is the...
-
Kroger Co. is one of the largest retail food companies in the United States as measured by total annual sales. The Kroger Co. operates supermarkets, convenience stores, and manufactures and processes...
-
time complexity of the following algorithm forn-1 to n-1 do for je +1 to n do Print & for Kn-3 to n+4 do print k
-
There are various compounds and epoxies that have been used to "final bed" rifle stocks. In your opinion, which is the best and why? Does this depend on the material of the stock? If so, why? Conduct...
-
Taylor Series: Problem 3 Previous Problem Problem List Next Problem 8 (5 points) Write the Taylor series for f(x) = x about x = 2 as C, (x 2)". Find the first five coefficients. n=0 - Co= C1= C2= C3=...
-
Why might the unit cost of those items started and completed during the period differ from the unit cost of all items completed and transferred during the period?
-
Show that if A is any m n matrix, then Im A = A and AIn = A.
-
Show how one can work backwards from the target step-by-step to the "present situation" in each of the following real-life problems. Target: Successfully financing a college education. Present...
-
Give the product expected, if any, when 2- methyl-2- propanol (or other compound indicated) reacts with each of the following reagents. (a) concentrated aqueous HCI (b) CrO3, in pyridine (c) H2SO4,...
-
Give the structure of a compound that satisfies the criterion given in each case. (There may be more than one correct answer.) An alcohol that, after acid-catalyzed dehydration. yields an alkene...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


