Explain each of the following observations. (a) The optical rotations of alanine are different in water, 1
Question:
Explain each of the following observations.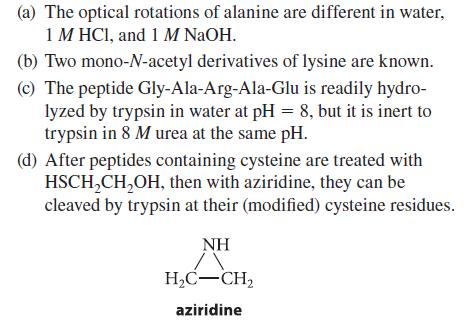
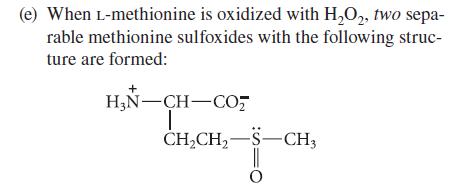
Transcribed Image Text:
(a) The optical rotations of alanine are different in water, 1 M HCl, and 1 M NaOH. (b) Two mono-N-acetyl derivatives of lysine are known. (c) The peptide Gly-Ala-Arg-Ala-Glu is readily hydro- lyzed by trypsin in water at pH = 8, but it is inert to trypsin in 8 M urea at the same pH. (d) After peptides containing cysteine are treated with HSCH₂CH₂OH, then with aziridine, they can be cleaved by trypsin at their (modified) cysteine residues. NH H₂C-CH₂ aziridine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a b c d Alanine like the other amino acids is a different compound in HCI NaOH and neutral HO because its ionization state is different and different ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
List and explain three (3) reasons why it is important to comply with the Privacy Act (1988) and the Australian Privacy S Principles when gathering information on children and when writing and...
-
This Comprehensive Problem is to acquaint you with the content of the 2009 financial state-ments of Home Depot, Inc. , reproduced in Appendix A of this textbook. (The 2009 financial statements are...
-
This Comprehensive Problem is to acquaint you with the content of the 2012 financial statements of Home Depot, Inc., reproduced in Appendix A of this textbook. (The 2012 financial statements are for...
-
Jane Grimes, retail fruit and vegetable merchant, does not keep a full set of accounting records. However, the following information has been produced from the business's records: 1. Summary of the...
-
What type of depreciation expense pattern is used under each of the following methods and when is its use appropriate? a. The straight-line method. b. The units-of-production method. c. The...
-
What is the difference between functional and dysfunctional conflict, and how does each affect performance?
-
Your first child is now a 1-year-old. Tuition currently costs $60,000 to attend a public college for four years. If these costs rise 5 percent annually, how much must you invest each year to cover...
-
The top prize for the state lottery is $100,000,000. You have decided it is time for you to take a chance and purchase a ticket. Before you purchase the ticket, you must decide whether to choose the...
-
Fast Feet Inc makes running shoes and they have gathered the following data for the month of October: \ table [ [ , Data ] , [ Cash on 1 0 / 1 , $ 1 6 , 0 0 0
-
When a mixture of the amino acids Phe and Gly is subjected to chromatography in a pH 6 buffer on the ion-exchange resin shown in Eq. 27.9, the Phe emerges from the column much later than the Gly,...
-
(a) What reagent would be used to convert the corresponding chloromethyl polystyrene resin into the following resin? (b) To a column containing this resin suspended in a pH 6 buffer is added a...
-
What are the four tools for planned experimentation? Give an example of the specific application of each of the tools in a study. LO4
-
Alec is an employee who drives a 2021 Ford C-Max with a fair-market value of $32,000. He has been given the choice to have the fringe benefit reported on his W-2 either using the lease-value rule or...
-
What resource do most thinking and learning technologies rely on to be effective? a) data b) images c) gas d) robots
-
Financial Reporting Problem Marks and Spencer plc (M&S) The financial statements of M&S (GBR) are presented in Appendix A. The companys complete annual report, including the notes to the...
-
Totally Chemical is considering an investment decision project in which the organization expands into the trucking business. Totally Chemical wants to begin this investment decision project by buying...
-
Presented below is the balance sheet of Sandhill Corporation as of December 31, 2017. SANDHILL CORPORATION BALANCE SHEET DECEMBER 31, 2017 Goodwill (Note 2) Buildings (Note 1) Inventory Land Accounts...
-
Zero Turbulence Airline provides air transportation services between Los Angeles, California, and Kona, Hawaii. A single Los Angeles to Kona round-trip flight has the following operating statistics:...
-
Prove the formula for (d/dx)(cos-1x) by the same method as for (d/dx)(sin-1x).
-
Classify each of the labeled bonds in the following structure in terms of the bond type ( or ) and the component orbitals that overlap to form the bond. (For example, the carbon--carbon bond in...
-
Give the structure of: 2E,7Z)-5-[(4- 1-propenyl]-2,7 -non adiene Be sure to read Study Guide Link 4.2 if you have difficulty with this problem.
-
Starting with the same two alkenes, would the products be different if DBr were used? Explain.
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


