When a mixture of the amino acids Phe and Gly is subjected to chromatography in a pH
Question:
When a mixture of the amino acids Phe and Gly is subjected to chromatography in a pH 6 buffer on the ion-exchange resin shown in Eq. 27.9, the Phe emerges from the column much later than the Gly, even though the two amino acids have the same isoelectric point. Explain.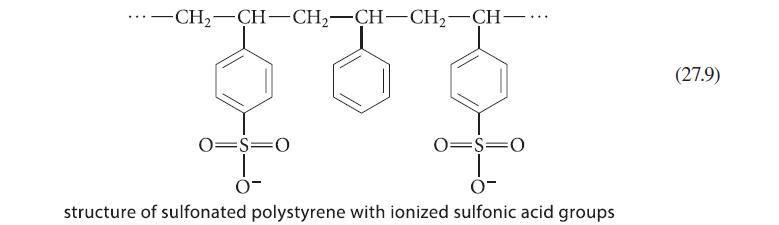
Transcribed Image Text:
-CH₂-CH-CH₂-CH-CH₂-CH-... 0=S=0 0- 0=S=O structure of sulfonated polystyrene with ionized sulfonic acid groups (27.9)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The resin contains a large number of pendant phenyl groups Phenylalanine also contains a phenyl grou...View the full answer

Answered By

Cristine kanyaa
I possess exceptional research and essay writing skills. I have successfully completed over 5000 projects and the responses are positively overwhelming . I have experience in handling Coursework, Session Long Papers, Manuscripts, Term papers, & Presentations among others. I have access to both physical and online library. this makes me a suitable candidate to tutor clients as I have adequate materials to carry out intensive research.
4.90+
1538+ Reviews
3254+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) What reagent would be used to convert the corresponding chloromethyl polystyrene resin into the following resin? (b) To a column containing this resin suspended in a pH 6 buffer is added a...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
LOCATE APPROPRIATE CPT CODES ICD-10-CM (CPT) FOR PROCEDURES BELOW- Outpatient procedures only 1. INCISION AND DRAINAGE OF A CYST --- 2. DEBRIDEMENT - 3. SIMPLE REPAIR OF A SUPERFICIAL WOUND- 4....
-
Over what period should an addition to an existing long-lived asset be depreciated? Explain.
-
Does an organization you work for or have worked for have any of the six characteristics of innovative cultures? Overall, does the organization have a creative culture?
-
You have accumulated $325,000 in a retirement account and continue to earn 8 percent on invested funds. a) What amount may you withdraw annually starting today based on a life expectancy of 20 years?...
-
Kyle, a single taxpayer, worked as a free-lance software engineer for the first three months of 2017. During that time, he earned $44,000 of self-employment income. On April 1, 2017 Kyle took a job...
-
On January 1, $34.000 cash is borrowed from a bank in return for a 8% Installment note with 36 monthly payments of $1,065 cach. (1) Prepare an amortization table for the first three months of this...
-
When either Norvir or Crixivan bind to the active site of HIV protease, the OH group in the middle of each molecule is found by X-ray crystallography to displace the tightly bound water present in...
-
Explain each of the following observations. (a) The optical rotations of alanine are different in water, 1 M HCl, and 1 M NaOH. (b) Two mono-N-acetyl derivatives of lysine are known. (c) The peptide...
-
How is sensitivity analysis used in capital budgeting?
-
The requirement for extended disclosures for oil and gas reserves described in Chapter 2 followed a Congressional hearing on the poor disclosures that Shell Oil had for its reserves. A.Explain three...
-
Question 9 Big Data techniques implemented in the financial sector include: fraud detection O marketing email campaign O customer relationship management techniques O inventory analysis
-
Problem 8-19A Attaining notfonpmt entity variances The Redmond Management Association held its annual public relations luncheon in April Year 2. Based on the previous year's results, the organization...
-
Kay, who is not a real estate dealer, sold an apartment house to Polly during the current year (2020). The closing statement for the sale is as follows. Total selling price $190,000 Add: Polly's...
-
1 English Writing Requirement Assignment Guidelines Sem 1 2023-24 Subject code AAE1D02 Subject title Introduction to Space Exploration Credit value 3 CAR Teachers Prof. WEN Chih-Yung, Prof. WU Bo,...
-
a. If Canace Company, with a break-even point at $960,000 of sales, has actual sales of $1,200,000, what is the margin of safety expressed (1) in dollars and (2) as a percentage of sales? b. If the...
-
A parking lot charges $3 for the first hour (or part of an hour) and $2 for each succeeding hour (or part), up to a daily maximum of $10. (a) Sketch a graph of the cost of parking at this lot as a...
-
A confused chemist Al Keane used the following names in a paper about alkenes. Although each name specifies a structure, in some cases the name is incorrect. Correct the names that are wrong. (a)...
-
Specify the configuration (E or Z) of each of the following alkenes. Note that D is deuterium, or 2H, the isotope of hydrogen with atomic mass = 2. (a) (b) CH3 CH,
-
Classify the compounds within each of the following pairs as either identical molecules (I), constitutional isomers (C), stereoisomers (S), or none of the above (N). (a) cyclopentane and cyclopentene...
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


