Explain how the compounds within each set can be distinguished using only UV spectroscopy. (a) 2-cyclohexenone and
Question:
Explain how the compounds within each set can be distinguished using only UV spectroscopy. 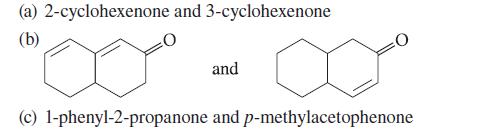
Transcribed Image Text:
(a) 2-cyclohexenone and 3-cyclohexenone (b) and (c) 1-phenyl-2-propanone and p-methylacetophenone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a b c The double bonds in ...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain how the compounds within each set can be distinguished using only UV spectroscopy. 2-cyclohexenone and 3-cyclohexenone
-
Assume you have unlabeled samples of the compounds within each of the following sets. Explain how UV-vis spectroscopy could be used to distinguish each compound in the set from the other (s). (a) (b)...
-
Explain how you would distinguish the compounds within each set by a simple chemical test with readily observable results, such as solubility in acid or base, evolution of a gas, and so forth....
-
Tom Lamont, age 30, and Lin Lamont, age 31, have been married for six years. They got married right after Tom graduated from college. They have come to you for help in planning their financial...
-
Laughter Landscaping has the following independent cases at the end of the year on December 31, 2014. a. Each Friday, Laughter pays employees for the current weeks work. The amount of the weekly...
-
Topple Corporation leases skyscrapers in cities throughout the world to large corporations. Topple's cost of debt financing is 9%, its cost of equity is 12%, and its tax rate is 35%. Required:...
-
Think about your last purchase either online or in a retail store. Were you presented with a nudge? If yes, what was the nudge? Did you make the purchase? If not, which item could have been presented...
-
IPO Pricing the Eyetech IPO was underpriced by about 54 percent. Should Eyetech be upset at Merrill Lynch over the under-pricing?
-
The provisions of the BNA Act, 1867 were relatively unique and distinct, designed by its colonists to address the country's particular circumstances. Select one: True False
-
The 13 C NMR spectrum of 2-ethylbutanal consists of the following absorptions: 11.5, 21.7, 55.2, and 204.7. Draw the structure of this aldehyde, label each chemically nonequivalent set of...
-
Outline two Wittig alkene syntheses of 2-methyl-1-hexene. Is one synthesis preferred over the other? Why?
-
What is the purpose of using the phrase \(x\) component of when describing some physical quantities?
-
Presented below is information related to Rembrandt Inc's inventory, assuming Rembrandt uses lower-of-LIFO cost- or-market. (per unit) Skis Boots Parkas Historical cost $190.00 $106.00 $53.00 Selling...
-
7. Below is a UML model describing a typical organization of class modules taken by students: A student can take several modules. (Note that a module is offered even if it is not taken by any...
-
Beswick Limited manufactures mountain and road bikes. The trial balance at 3 1 December 2 0 2 0 was as follows: Dr Cr Revenue 3 , 5 6 4 , 3 0 0 Purchases 1 , 5 7 8 , 2 5 0 Inventory on 3 1 / 1 2 / 1...
-
Kubin Company's relevant range of production is 10,000 to 12,000 units. When it produces and sells 11,000 units, its average costs per unit are as follows: Average Cost per Unit $ 7.10 Direct...
-
Smithen Company, a wholesale distributor, has been operating for only a few months. The company sells three products-sinks, mirrors, and vanities. Budgeted sales by product and in total for the...
-
Two n n matrices A, B are said to be simultaneously diagonalizable if there is a nonsingular matrix S such that both S-1 A S and S-1 B S are diagonal matrices. (a) Show that simultaneously...
-
You are the newly appointed tax practitioner to complete Emilys tax return and have downloaded the prefill report for Emilys tax return (hint, you can read what a prefill report is here (Links to an...
-
Compound A has the molecular formula C6H12O3 and shows a strong IR absorption peak at 1710 cm-1. When treated with iodine in aqueous sodium hydroxide, A gives a yellow precipitate. When A is treated...
-
The following is an example of a reaction sequence developed by Derin C. D'Amico and Michael E. Jung (UCLA) that results in enantiospecific formation of two new chirality centers and a carbon-carbon...
-
Additional evidence for the halogenation mechanisms that we just presented comes from the following facts: (a) Optically active 2-methyl-1-phenylbutan-1-one undergoes acid-catalyzed racemization at a...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


