Explain why the hydration of ethylene (Eq 4.44) is a very slow reaction. (Think about the structure
Question:
Explain why the hydration of ethylene (Eq 4.44) is a very slow reaction. (Think about the structure of the reactive intermediate and apply Hammond’s postulate.)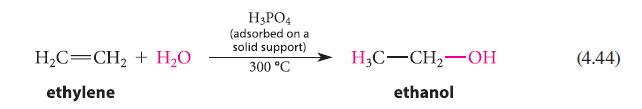
Transcribed Image Text:
H₂C=CH₂ + H₂O ethylene H3PO4 (adsorbed on a solid support) 300 °C H3C-CH₂-OH ethanol (4.44)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The intermediate in the hydration of ethylene is a primary carbocation Hence the transition s...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain why the hydration of this alkene occurs 1015 times faster than the hydration ofethene: OH H20 CH,CH,OCHCH3 CH.CH,OCH=CH, H,SO,
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Administrative agencies, boards or commissions usually use registration or licensing to control business subject to the administrative law. True False
-
A new truck, manufactured by General Motors Corp. (GMC), stalled in rush hour traffic on a busy interstate highway because of a defective alternator, which caused a complete failure of the trucks...
-
The energy from radiation can be used to cause the rupture of chemical bonds. A minimum energy of 941 KJ/mol is required to break the nitrogen-nitrogen bond in N2. What is the longest wavelength of...
-
Find the probability of randomly selecting a school with 600 or more students.
-
Horne executed a $100,000 note in favor of R. C. Clark. On the back of the instrument was a restriction stating that the note could not be transferred, pledged, or otherwise assigned without Hornes...
-
Which of the following are items which are likely to need a prepayment adjustment at the year end? Select one: A. Electricity B. Bank C. Motor vehicles D. Rent received
-
Give the structures and IUPAC substitutive names of the isomeric alkenes with molecular formula C 6 H 12 containing five carbons in their principal chains.
-
Give the mechanism for the reaction in Eq. 4.43. Show each step of the mechanism separately with careful use of the curvedarrow notation. Explain why the rearrangement takes place. H HC-C-CH=CH + HO...
-
Wanting to finalize a sale before year-end, on December 29, WR Outfitters sold to Bob a warehouse and the land for $125,000. The appraised fair market value of the warehouse was $75,000, and the...
-
Alvarado Company produces a product that requires 5 standard direct labor hours per unit at a standard hourly rate of $12.00 per hour. If 5,700 units used 29,400 hours at an hourly rate of $11.40 per...
-
7. (30 points) You are a teaching assistant (TA) for a new course in the department and you wish to measure the amount of time that students spend engaging with the online resources. Using the Canvas...
-
Mod Clothiers makes women's clothes. It costs $28,000 to produce 5,000 pairs of polka-dot polyester pants. They have been unable to sell the pants at their usual price of $50.00. The company is...
-
In a mid-sized manufacturing company, the annual financial statements were prepared for audit by an external auditing firm. The company\'s finance team had diligently compiled the financial data, and...
-
Explain the meaning of the SMART acronym. In 100-200 words, define what the words "goal" and "success" mean to you. Summarize your thoughts on whether or not the SMART model can help you become a...
-
Distinguish between reticular and elastic connective tissues.
-
Use critical values to test the null hypothesis H0: 1 2 = 20 versus the alternative hypothesis H0: 1 2 20 by setting a equal to .10, .05, .01, and .001. How much evidence is there that the...
-
2, 3-Dimethyl-2, 3-butanediol has the common name pinacol. On heating with aqueous add, pinacol rearranges to pinacolone, 3, 3-dimethyl-2-hutanone. Suggest a mechanism for this reaction. CH :-C CH ...
-
As a rule, axial alcohols oxidize somewhat faster than equatorial alcohols. Which would you expect to oxidize faster, cis-4-tert-butylcyclohexanol or trans-4-tert-butylcyclohexanol? Draw the more...
-
Propose a synthesis of bicyclohexylidene, starting from Cyclohexanone as the only source of carbon. Bicyclohexylidene
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


