Give the mechanism for the reaction in Eq. 4.43. Show each step of the mechanism separately with
Question:
Give the mechanism for the reaction in Eq. 4.43. Show each step of the mechanism separately with careful use of the curvedarrow notation. Explain why the rearrangement takes place.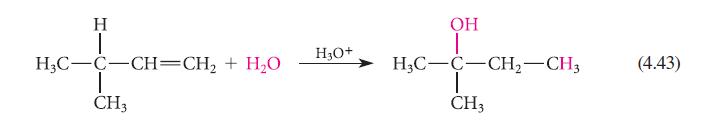
Transcribed Image Text:
H H₂C-C-CH=CH₂ + H₂O CH3 H3O+ OH H3C-C-CH₂-CH3 I CH3 (4.43)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
In the first step of the reaction the double bond is protonated by the acid catalyst in a Brnsted ...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the mechanism for the last step in the biosynthesis of isopentenyl pyrophosphate, showing why ATP is required.
-
The rate law for the reaction 2NO2Cl(g) 2NO2(g)+ Cl2(g) (overall equation) is first order in nitryl chloride, NO2Cl. Rate = k[NO2Cl] Explain why the mechanism for this reaction cannot be the single...
-
A sample of lactic acid (CH 3 CH(OH)COOH) was extracted from a natural source and found to be optically active. It was then subjected to two reactions, as shown below. Sample 1 was optically active...
-
Find the exact value of sin(x - y) if sin(x) = 3T 3T T
-
At approximately 7:50 p.m., bells at the train station rang and red lights flashed, signaling an express trains approach. David Harris walked onto the tracks, ignoring a yellow line painted on the...
-
A diode laser emits at a wavelength of 987 nm. (a) In what portion of the electromagnetic spectrum is this radiation found? (b) All of its output energy is absorbed in a detector that measures a...
-
Find the probability of randomly selecting a school with between 300 and 999 students, inclusive. In Exercises 3740, use the Pareto chart, which shows the results of a survey in which 874 adults were...
-
Gator Beach Marts, a chain of convenience grocery stores in the Fort Lauderdale area, has store hours that fluctuate from month to month as the tourist trade in the community varies. The utility...
-
Check my work 4 Part 2 of 4 Required information Exercise 6-4A Calculate inventory amounts when costs are rising (L06-3) (The following information apples to the questions displayed below.) During...
-
Explain why the hydration of ethylene (Eq 4.44) is a very slow reaction. (Think about the structure of the reactive intermediate and apply Hammonds postulate.) HC=CH + HO ethylene H3PO4 (adsorbed on...
-
(a) Give the structures of five alkenes, each with the formula C 6 H 12 , that would give hexane as the product of catalytic hydrogenation. (b) How many alkenes containing one double bond can react...
-
Defendants who are citizens of the United Kingdom and have played, taught, coached, and worked in the soccer industry most of their lives were hired by plaintiffs to provide soccer camp services to...
-
Small town Diners has a policy of treating dividends as a passive residual. It forecasts that net earnings after taxes in the coming year will be $500,000. The firm has earned the same $500,000 for...
-
Part 1-Chi-Square Goodness-of-Fit Tests A health psychologist was interested in women's workout preferences. Of the 56 participants surveyed, 22 preferred running, 8 preferred swimming, 15 preferred...
-
The Campbell Company is considering adding a robotic paint sprayer to its production line. The sprayer's base price is $1,070,000, and it would cost another $21,000 to install it. The machine falls...
-
Problem 1. (10 points) Consider the space X = R22 and the map L XX defined as traceX -traceX L:X X = X 0 0 1. Show that L is a linear map; 2. Find the matrix representation M = mat L in the canonical...
-
Suppose that the exchange rate is 1.25 = 1.00. Options (calls and puts) are available on the Philadelphia exchangein units of10,000 with strike prices of $1.60/1.00. Options (calls and puts) are...
-
Explain why injured dense regular connective tissue and cartilage are usually slow to heal.
-
Cobb Manufacturing Company uses a process cost system and average costing. The following production data is for the month of June 2011. Production Costs Work in process, beginning of the month:...
-
A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate:...
-
Identify the reagents a?f in the following scheme: .Br CH-
-
Galactose, a constituent of the disaccharide lactose found in dairy products, is metabolized by a pathway that includes the isomerization of UDP-galactose to UDP-glucose, where UDP = uridylyl...
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


