Give a curved-arrow mechanism for the following electrophilic substitution reaction. -H DSO4 -D
Question:
Give a curved-arrow mechanism for the following electrophilic substitution reaction.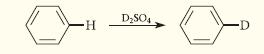
Transcribed Image Text:
-H D₂SO4 -D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Construct the mechanism in terms of the three steps given in this section Step 1 In this reaction a ...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The total amount paid in by__________for the shares they purchase is described as common stock. Select answer from the options below creditors bondholders employees stockholders
-
2. Draw the curved arrow mechanism for the reaction between 2-methoxyphenoxide and 3-chloro-1,2-propanediol, in the guaifenesin synthesis. Be sure to clearly show the bimolecular transition state.
-
Provide the curved arrow mechanism for the following reaction. tetraline OH SH S %3D 200 C N' hypoxanthine phosphorous pentasulfide 6-mercaptopurine
-
Which of the following activity bases would best be used to allocate setup activity to products? a. Number of inspections b. Direct labor hours c. Direct machine hours d. Number of production runs
-
Revenue recognition Neiman Marcus, a U.S. retailer, uses the accrual basis of accounting and follows U.S. GAAR It recognizes revenue at the time it sells merchandise. Indicate the amount of revenue...
-
What are the emf and internal resistance of the battery in FIGURE P28.46? Open: 1.636 A A Closed: 1.565 A 50 100 FIGURE P28.46
-
What is the probability that a voter chosen at random did not vote for a Democratic representative in the 2008 election? (Source: Federal Election Commission) Using a Frequency Distribution to Find...
-
James Williams, a star college basketball player, received a contract from the Midwest Blazers to play professional basketball. The contract calls for a salary of $420,000 a year for four years,...
-
Yole Compeny menufactures hair brushes thet sell st wholesole for $ 3 per unit. The company had no beginning inventory In the prior yesr. These dats summorize the current and prior year operstions:...
-
Outline a synthesis of p-bromonitrobenzene from benzene.
-
This problem describes the result that established the intrinsic preference for 1,2-addition in the reaction of hydrogen halides with conjugated dienes. (a) What is the relationship between the...
-
Lewis and Associates has been in the termite inspection and treatment business for five years. The following is a list of accounts for Lewis on June 30, 2012. It reflects the recurring transactions...
-
A carload of Hg-ore containing grains of cinnabar (86%Hg by mass; density = 8.19 g/cm3) and grains of basalt (containing no Hg; density=2.84 g/cm3) is to be sampled and analyzed for mercury. The...
-
CMS reviews acute IPPS and long-term care hospital (LTCH) records for payment purposes. Documentation and coding assignment must be accurate and specific. CMS contracts with Medicare Administrative...
-
Problem 2. x3+2x+1 f(x) = = 5-x 8H xx (4 points) Without graphing the function, find the limits lim f(x) and lim f(x) analyt- ically and show your work. Specify if the limits are - or +. (1 point)...
-
For change management, answer the following questions in detail, citing some industry examples: 1. What would you do if your manager requested you change your way of working on a project? 2. What do...
-
1.Sony has just released a new CD recording (okay, not new because we don't buy CDS) but anyway.Here is some cost and price information: CD Disc and Packaging (material and labor) $1.75/CD...
-
Under what circumstances might a company write down its inventory to carrying value below cost?
-
When is the indirect pattern appropriate, and what are the benefits of using it?
-
Characterize each step of the mechanism in Eq. 18.42b in terms of the fundamental processes discussed in the previous section. Give the electron count and the oxidation state of the metal in each...
-
What two sets of aryl bromide and alkene starting materials would give the following compound as the product of a Heck reaction? C'
-
What product is expected when cyclopentene reacts with iodobenzene in the presence of tri-ethlamine and a Pd(0) catalyst?
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...

Study smarter with the SolutionInn App


