Give curved-arrow mechanisms for the reactions given in Fig. P19.64. (b) (c) S PhCPh (CH3O) 3P: +
Question:
Give curved-arrow mechanisms for the reactions given in Fig. P19.64.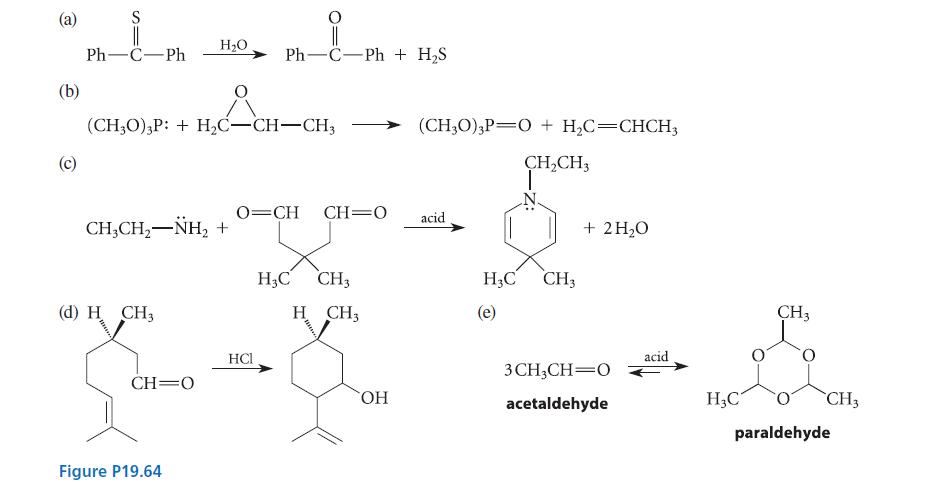
Transcribed Image Text:
(b) (c) S Ph—C−Ph (CH3O) 3P: + H₂C-CH-CH3 CH,CH,NH, + (d) H CH3 H₂O CH O Figure P19.64 Ph-C-Ph + H₂S HCI O=CH CH O acid H3C CH3 H CH3 (CH3O) 3P O + H₂C=CHCH3 CH₂CH3 OH H3C CH3 (e) + 2H₂O 3CH₂CH=0 acetaldehyde acid H₂C CH3 CH3 paraldehyde
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 5 PhCPh H0 PhCPh COH H OH HOH PhCPh PhC Ph PhC Ph H SH SH PhCPh OH PhCPh HS b Opening of the epoxi...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw curved-arrow mechanisms for the reactions given in Fig. P18.73.
-
Draw curved-arrow mechanisms for the reactions given in Fig. P18.78. (a) (b) N=C Figure P18.78 -F + H-N: -OH(CH3)2C=CH 95 C, 6 h DMSO HSO4 N=C- (CH3)3C- H ob. -OH F-
-
Write out the steps in the reaction mechanisms for the reactions given in eq. 8.20. CHO_ HOCH2CH2OCH, 2-methoxyethanol H+ CH-CH HOCHCH OH HOCH CH2OCH CH OH diethylene glycol
-
Runnals National Bank has experienced the following trends over the past five years (all figures in millions of dollars): Input Area: 1 2 3 4 5 Net Income (after tax) 2.65 2.75 3.25 3.65 4.00 Total...
-
Todd McKinney Magic Shows accounting records include the following account balances as of December 31: During 2012, the business recorded the following: a. Prepaid annual rent of $8,000. b. Made the...
-
On August 15, Blockstar Company buys 2,100 shares of Netvision common stock for $48,000, plus brokerage fees of $300. On October 31, Blockstar sells 500 shares of Netvision stock for $12,500, less...
-
Horse pregnancies. Bigger animals tend to carry their young longer before birth. The length of horse pregnancies from conception to birth varies according to a roughly Normal distribution with mean...
-
Sheldon Optics produces medical lasers for use in hospitals. The accounts and their balances appear in the ledger of Sheldon Optics on October 31 of the current year as follows: Preferred 2% Stock,...
-
8. Risk and return Suppose Janet is choosing how to allocate her portfolio between two asset classes: risk-free government bonds and a risky group of diversified stocks. The following table shows the...
-
Identify the following compounds. (a) C0H0O NMR: 8 2.82 (6H, s), 8 8.13 (4H, s) IR: 1681 cm-, no 0-H stretch (b) C-H0ONMR: 8 9.8 (1H, s), 8 1.1 (9H, s) NMR in Fig. P19.66 (p. 1002) IR: 1701 cm-, 970...
-
Thumbs Throckmorton, a graduate student in his twelfth year of study, has designed the synthetic procedures shown in Fig. P19.63. Indicate the problems (if any) that each synthesis is likely to...
-
Name the two major components of matrix and, if applicable, subclasses of each component.
-
An employer has calculated the following amounts for an employee during the last week of June 2021. Gross Wages $1,800.00 Income Taxes $414.00 Canada Pension Plan $94.00 Employment Insurance $28.00...
-
Section Two: CASE ANALYSIS (Marks: 5) Please read the following case and answer the two questions given at the end of the case. Zara's Competitive Advantage Fashion houses such as Armani and Gucci...
-
The activity of carbon in liquid iron-carbon alloys is determined by equilibration of CO/CO2 gas mixtures with the melt. Experimentally at PT = 1 atm, and 1560C (1833 K) the equilibrated gas...
-
Apply knowledge of concepts and theories covered in the course to leader - the leader can either be themselves if they lead a team, someone real and personally known to them (such as a boss or leader...
-
A resistor in a dc circuit R = 1.2 2. The power dissipated P is a second-degree function of the voltage V. Graph P versus V from V = 0.0 V to V = 3.0 V.
-
Let (i). Write down necessary and sufficient conditions on the entries a, b, c, d that ensures that A has only real eigenvalues. (ii).Verify that all symmetric 2 2 matrices satisfy your conditions....
-
Prove the following D,(cos x) = - sin x (Hint: Apply the identity cos(A + B) = cos A cos B sin A sin B)
-
Triphenylphosphine can be used to convert epoxides to alkenes-for example, Propose a likely mechanism for this reaction. + (CgHs)3PO
-
When benzaldehyde reacts with a peroxy acid, the product is benzoic acid. The mechanism for this reaction is analogous to the one just given for the oxidation of acetophenone, and the outcome...
-
Which compound in each of the following pairs has the higher boiling point? (Answer this problem without consulting tables.) (a) Pentanal or 1-pentanol (b) 2-Pentanone or 2-pentanol (c) Pentane or...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


