Give the common name for each of the following compounds, and tell whether each is a primary,
Question:
Give the common name for each of the following compounds, and tell whether each is a primary, secondary, or tertiary alkyl halide.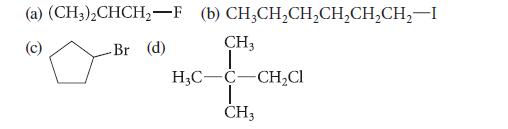
Transcribed Image Text:
(a) (CH3)₂CHCH₂-F -Br (d) (b) CH3CH₂CH₂CH₂CH₂CH₂2-I CH3 T H₂C-C-CH₂Cl I CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a Isobutyl fluoride is a primary alk...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw all the isomers that have the molecular formula C5H11Br(Hint: There are eight such isomers.) a. Give the systematic name for each of the isomers. b. Give a common name for each isomer that has...
-
Give an IUPAC systematic or common name for each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) CH3CN Cl NH2
-
Give the IUPAC name for each of the following alkyl groups, and classify each one as primary, secondary, or tertiary: (a) CH3(CH2)10CH2-- (b) (c) --C(CH2CH3)3 (d) (e) (f) -CH2CH2CHCH2CH2CH3 CH2CH3...
-
What are the costs of healthcare, where does the money come from, and where is it spent?
-
Could the company be guilty of motor vehicle homicide?
-
The cross section of an unbalanced wide-flange beam is shown in the figure. Derive the following formula for the distance h1 from the centerline of one flange to the shear center S: Also, check the...
-
Suppose you work for a major trading company exporting timber from Canada, petroleum from Britain, and processed food products from the United States. To enhance your career prospects, you want to...
-
Glenn Grimes is the founder and president of Heartland Construction, a real estate development venture. The business transactions during February while the company was being organized are listed...
-
Hexoble Budgeting and Variance Analysis I Love My Chocolate Company makes dark chocolate and light chocolate. Both products require cocoa and sugar The following planning information has been made...
-
Give the structure of each of the following compounds. (a) 2,2-dichloro-5-methylhexane (b) Chlorocyclopropane (c) 6-bromo-1-chloro-3-methylcyclohexene (d) Methylene iodide
-
Provide an IUPAC substitutive name for each of the following compounds. (a) CH3CHCHCH3 (b) CH3CHCH=CHCHCH3 | T | CHCHSH
-
Solve Problem 17.5 but assume that m = 0.6 for all stations. Other data are the same. Problem 17.5 An eight-station assembly machine has an ideal cycle time of 6 sec. The fraction defect rate at each...
-
Based on the reading,How to make sure your next product or service launch drives growth (click the underlined link),what stands out to you as the most important factor in a differentiated launch...
-
OM in the News has previously looked at the Waffle House Index, used to measure the damage from hurricanes. The index made the news again for Hurricane Ian. According to the Boston Globe, 40 Waffle...
-
Mixture of persuasive and negative formal I am Elizabeth grinderFirst part email Next part setting up the meeting Final part memo The reader is Robert * do not come off accusatory*** Project TWO:...
-
Anyone who has sampled today's social media offerings has probably experienced this situation: You find a few fascinating blogs, a few interesting people to follow on Twitter, a couple of podcast...
-
a. Begin with a converging lens of focal length f. Place an illuminated object a distance p, in front of the lens. For all positive values of p;: 1. calculate and sketch a graph of the location of...
-
Describe the three types of neurons classified on the basis of structure.
-
Wilsons Auto Repair ended 2011 with Accounts Receivable of $85,000 and a credit balance in Allowance for Uncollectible Accounts balance of $11,000. During 2012, Wilsons Auto Repair had the following...
-
Convert the following representation of ethane, C2H6. Into a conventional drawing that uses solid, wedged, and dashed lines to indicate tetrahedral geometry around each carbon (gray = c, ivory =H).
-
What are likely formulas for the following substances? (a) GeCl? (b) AlH? (c) CH? Cl2 (d) SiF? (e) CH3NH?
-
Write line-bond structures for the following substances, showing all nonbonding electrons: (a) CHCl3, chloroform (b) H2S, hydrogen sulfide (c) CH3NH2, methylamine (d) CH3Li,methyllithium
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


