Give the major product formed when each of the following amines is treated exhaustively with methyl iodide
Question:
Give the major product formed when each of the following amines is treated exhaustively with methyl iodide and then heated with Ag2O. Explain your reasoning.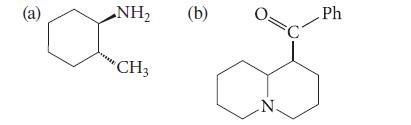
Transcribed Image Text:
(a) NH₂ CH3 (b) N. C Ph
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a The product of the exhaustive methylation followed by heatin...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Given the major product formed when each of the following amines is treated exhaustively with methyI iodide and then heated with Ag2O.Explain your reasoning. Ph N.
-
Give the major product formed when each of the following alcohols is heated in the presence of H2SO4; a. b. c. d. e. f. CH3CH2CH2CH2CH2OH CH CH3CH2C CHCH OH CH3 CHCH2CH3 OH CH,OH CH3 CH,CH2CH CCH OH...
-
Draw the major product(s) that are expected when each of the following amines is treated with excess methyl iodide and then heated in the presence of aqueous silver oxide. (a) (b) NH2 NH2
-
Your friend has just started a retail clothing store in kamloops. She will be purchasing inventory to make her own clothing (One Style) and she will also be buying ready to sell iteams. She has come...
-
Identify the three types of ownership structures and discuss the advantages and disadvantages of each.
-
What kinds of campus activities could a full-time student do that might lead to the perception that he or she is a charismatic leader? In pursuing those activities, what might the student do to...
-
What factors might a business take into account when deciding between preference shares and loan capital as a means of raising new finance?
-
Stabler Co.s projected March 31, 2011, balance sheet follows. Additional information about the company is as follows: ¢ Expected sales for April and May are $240,000 and $260,000, respectively....
-
1. All of the following may be considered intangible assets except : Accounts receivable. Franchises. Copyrights. Goodwill. 2. When shares of stock are sold from one investor to another, they will...
-
Provide a reaction mechanism for the reaction shown in Eq. 23.36. NH 5- + 3 Br Br NH Br Br + 3HBr (23.36)
-
Suggest two syntheses of N-ethylcyclohexanamine by reductive amination.
-
At what cold-reservoir temperature (in C) would a Carnot engine with a hot-reservoir temperature of 427C have an efficiency of 60%?
-
Rosita Flores owns Rosita's Mexican Restaurant in Tempe, Arizona. Rosita's is an affordable restaurant near campus and several hotels. Rosita accepts cash and checks. Checks are deposited...
-
Your second task will require you to recover a payload from the conversation. Just need 2.3. Need you to explain step by step, and concept by concept if possible. Use wireshark. Tell me your answer...
-
2. Supply for art sketchbooks at a price of $p per book can be modelled by P <10 S(p) = = textbooks. p3+p+3 p 10 (a) What is the producer revenue at the shutdown point? (b) What is the producer...
-
Patterson Company produces wafers for integrated circuits. Data for the most recent year are provided: Expected Consumption Ratios Activity Driver Wafer A Wafer B Inserting and sorting process...
-
The elementary gas-phase reaction 2A + B C+D is carried out isothermally at 450 K in a PBR with no pressure drop. The specific reaction rate was measured to be 2x10-3 L/(mol-min-kgcat) at 50C and the...
-
Find the eigenvalues, to 2 decimal places, of the matrices in Exercise 10.6.18 by applying the QR algorithm to the tridiagonal form. In Exercise 10.6.18 Use Householder matrices to convert the...
-
Solve each equation. x 3 - 6x 2 = -8x
-
In the previous problem and the associated molecular model at the book's website, you considered the role of HOMOs and LUMOs in an SN2 reaction. (a) What is the LUMO in an SN1 reaction and in what...
-
SN2 reactions that involve breaking a bond to a chirality center can be used to relate configurations of molecules because the stereochemistry of the reaction is known. (a) Illustrate how this is...
-
Keeping in mind that carbocations have a trigonal planar structure, (a) Write a structure for the carbocation intermediate (b) Write structures for the alcohol (or alcohols) that you would expect...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


