Give the product(s) expected when d-mannose (or other compound indicated) reacts with each of the following reagents.
Question:
Give the product(s) expected when d-mannose (or other compound indicated) reacts with each of the following reagents. (Assume that cyclic mannose derivatives are pyranoses.)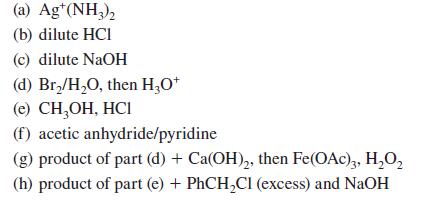
Transcribed Image Text:
(a) Ag¹(NH3)₂ (b) dilute HCI (c) dilute NaOH (d) Br₂/H₂O, then H3O+ (e) CH₂OH, HCI (f) acetic anhydride/pyridine (g) product of part (d) + Ca(OH)₂, then Fe(OAc)3, H₂O₂ (h) product of part (e) + PhCH₂Cl (excess) and NaOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Note that carboxylic acids derived from carbohydrates usually exist as l...View the full answer

Answered By

Rodrigo Louie Rey
I started tutoring in college and have been doing it for about eight years now. I enjoy it because I love to help others learn and expand their understanding of the world. I thoroughly enjoy the "ah-ha" moments that my students have. Interests I enjoy hiking, kayaking, and spending time with my family and friends. Ideal Study Location I prefer to tutor in a quiet place so that my students can focus on what they are learning.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the principal organic product(s) expected when 2- methylthiophene or other compound indicated reacts with each of the following reagents. (a) HNO3 (b) Dilute aqueous NaOH (c) Product of part (a)...
-
Give the principal organic product(s) expected when p-chloroaniline or other compound indicated reacts with each of the following reagents. (a) Dilute HBr (b) CH 3 CH 2 MgBr in ether (c) NaNO 2 ,...
-
Give the principal organic product(s) expected when 2- methylpyridine or other compound indicated reacts with each of the following reagents. (a) Diliute aqueous NaOH (b) HNO3, H2SO4, heat; then -OH...
-
Write a nonrecursive function that takes the first Node in a linked list as an argument and reverses the list, returning the first Node in the result.
-
Give the symbols for two other isotopes of (a) 14 N, (b) 56 Fe, and (c) 118 Sn
-
The following table shows an example of gross domestic product values for five countries over six years in equivalent U.S. dollars ($). a. How could you improve the readability of this table? b. The...
-
Officers The offices of president, vice president, secretary, and treasurer for an environmental club will be filled from a pool of 14 candidates. Six of the candidates are members of the debate...
-
Wichita University sells 4,000 season basketball tickets at $180 each for its 10 game home schedule. Give the entry to record (a) The sale of the season tickets (b) The revenue recognized for playing...
-
Mark and Mandy were pregnant with triplets; two girls and a boy. Mandy's due date was scheduled for December 28. After much frustration and anticipation on the morning of December 31, Mandy decided...
-
Give the products expected when D-ribose (or other compound indicated) reacts with each of the following reagents. (a) dilute HNO3 (b) -CN, HO (c) product of part (b) + H/Pd/BaSO4 + HO+/HO (d) CHOH,...
-
Consider the structure of cellobiose, a disaccharide obtained from the hydrolysis of the polysaccharide cellulose. Into what monosaccharide(s) is cellobiose hydrolyzed by aqueous HCl? HO CHOH -...
-
In Problems 544, solve each logarithmic equation. Express irrational solutions in exact form. 2 log5 x = 3 log5 4
-
For the data in Problem 42, how would you predict demand for medical kits using (a) moving averages and (b) exponential smoothing (with alpha values equal to 0.5 and greater) for the 21st week? Data...
-
For a light ray that crosses the interface between medium 1 having index of refraction \(n_{1}\) and medium 2 having index of refraction \(n_{2}\), what relationship between \(\theta_{1}\) and...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
Seniority quantum numbers typically measure how many fermions are in some sense "not paired" with another fermion. For the quasispin model of Problem 31.3 , define the Racah seniority $v$ through...
-
(a) Place a perfectly conducting sphere with radius a in a uniform electric field E 0 and let an origin centered electric dipole field represent the field produced by the sphere. Use this information...
-
What is the ground-state electron configuration of bromine (Br, atomic number 35)?
-
Solve each equation. x 3 - 6x 2 = -8x
-
When DNA is treated with 0.5 M NaOH at 25C, no reaction takes place, but when RNA is subjected to the same conditions, it is rapidly cleaved into mononucleotide 2- and 3-phosphates. Explain.
-
Indicate whether you would expect imidazole to have high or low water solubility, and why.
-
What product is expected when 4-(dimethylamino)pyridine reacts with CH3I?
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


