Give the products expected when D-ribose (or other compound indicated) reacts with each of the following reagents.
Question:
Give the products expected when D-ribose (or other compound indicated) reacts with each of the following reagents.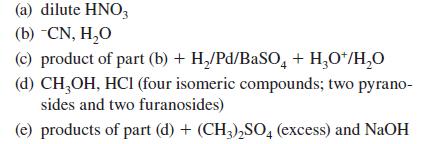
Transcribed Image Text:
(a) dilute HNO3 (b) -CN, H₂O (c) product of part (b) + H₂/Pd/BaSO4 + H₂O+/H₂O (d) CH₂OH, HCI (four isomeric compounds; two pyrano- sides and two furanosides) (e) products of part (d) + (CH3)₂SO4 (excess) and NaOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Note that carboxylic acids derived from carbohydrates usually exist as lactones and aldos...View the full answer

Answered By

Isaiah Mutinda
As a graduate with Bs in Maths and Computer Science and having worked as a freelance full stack software developer for 3 years running I believe I have what it takes to conformable tutor and mentor a student to a professional developer also.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the principal organic product(s) expected when 2- methylthiophene or other compound indicated reacts with each of the following reagents. (a) HNO3 (b) Dilute aqueous NaOH (c) Product of part (a)...
-
Give the principal organic product(s) expected when 2- methylpyridine or other compound indicated reacts with each of the following reagents. (a) Diliute aqueous NaOH (b) HNO3, H2SO4, heat; then -OH...
-
Give the principal organic product(s) expected when ethyl benzoate or the other compound indicated reacts with each of the following reagents. (a) HO, heat, acid catalyst (b) NaOH, HO (c) aqueous...
-
In the game of chess, if a board position is repeated three times with the same side to move, the side to move can declare a draw. Describe how you could test this condition using a computer program.
-
Calculate the binding energy and the binding energy per nucleon from the masses given in Table 40-1 for (a) 12 C, (b) 56 Fe, (c) 238 U. Table 40-1 Atomic Masses of the Neutron and Selected Isotopes...
-
Water flows through a horizontal pipe at a rate of 2.4 gal/s. The pipe consists of two sections of diameters 4 in and 2 in with a smooth reducing section. The pressure difference between the two pipe...
-
Jukebox You look over the songs on a jukebox and determine that you like 15 of the 56 songs. (a) What is the probability that you like the next three songs that are played? (Assume a song cannot be...
-
On January 1, 2017, Prasad SpA had the following equity accounts. Share Capital-Ordinary (25 par value, 48,000 shares issued and outstanding)....1,200,000 Share...
-
Shearers Foods, part of the $374 billion global snack food industry, employs 3,300 people in Brewster, Ohio. If Shearers purchased a packaging unit for $120,440 with a life expectancy of 687,000...
-
What product(s) would be obtained when cellulose is treated first exhaustively with dimethyl sulfate/NaOH, then with 1 M aqueous HCl?
-
Give the product(s) expected when d-mannose (or other compound indicated) reacts with each of the following reagents. (Assume that cyclic mannose derivatives are pyranoses.) (a) Ag(NH3) (b) dilute...
-
Use Taylors formula for (x, y) at the origin to find quadratic and cubic approximations of near the origin. (x, y) = ln (2x + y + 1)
-
The Buckle, Inc., operates 387 stores in 39 states, selling brand name apparel like Lucky jeans and Fossil belts and watches. Some of the items included in its 2008 statement of cash flows presented...
-
Assume that on July 1, 2011, Big Corp. loaned Little Corp. \(\$ 12,000\) for a period of one year at 6 percent interest. What amount of interest revenue will Big report for 2011? What amount of cash...
-
A vacuum column with 25 real stages is operating with a pressure drop of \(0.3 \mathrm{in}\). of water per stage. Assume pressure drop in the condenser and the reboiler is 0.6 in. of water each. The...
-
You want to determine the viscosity of an oil which has an SG of 0.9. To do this, you drop a spherical glass bead $(\mathrm{SG}=2.7)$ with a diameter of $0.5 \mathrm{~mm}$ into a large vertical...
-
Show that 673 - 356 can be computed by adding 673 to the 10's complement of 356 and discarding the end carry. Draw the block diagram of a three-stage decimal arithmetic unit and show how this...
-
What is the maximum possible value of the angular momentum for an outer electron in the ground state of a bromine atom?
-
Let (x) = x 2 - 9, g(x) = 2x, and h(x) = x - 3. Find each of the following. (((--) 2
-
Although the synthesis of heterocyclic rings was not discussed in the text, many such syntheses employ reactions that are similar or identical to reactions in other parts of the text. Give...
-
One theory of genetic mutation postulates that some mutations arise as the result of mispairing of bases in DNA caused by the existence of relatively rare isomeric forms of the bases. Show the...
-
The stability of a DNA double helix can be measured by its melting temperature, Tm, defined as the temperature at which the helix is 50% dissociated into individual chains. Which of the following...
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


