Ibuprofen is a drug sold as a nonprescription anti-inflammatory medication. (a) What are the concentrations of ibuprofen
Question:
Ibuprofen is a drug sold as a nonprescription anti-inflammatory medication.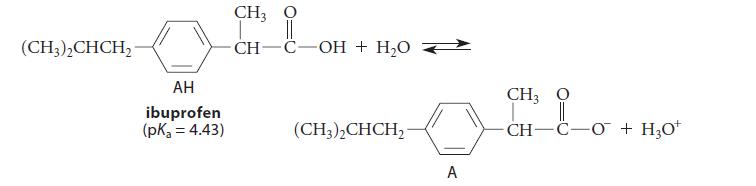
(a) What are the concentrations of ibuprofen and its conjugate base if 10–4 mole of ibuprofen is dissolved in an aqueous solution containing a large excess of a buffer at pH = 5.0?
(b) Ibuprofen is taken orally. What fraction of ibuprofen is dissociated in stomach acid? (Take the pH of stomach acid to be 2.0.)
(c) What is the dissociation state of ibuprofen in the bloodstream (pH = 7.4)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





