Electrostatic potential maps of (a) Formaldehyde (CH2O) and (b) Methanethiol (CH3SH) is shown. Is the formaldehyde carbon
Question:
Electrostatic potential maps of
(a) Formaldehyde (CH2O) and
(b) Methanethiol (CH3SH) is shown. Is the formaldehyde carbon atom likely to be electrophilic or nucleophiic? What about the Methanethiol sulfur atom?Explain.
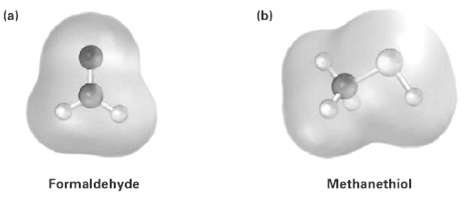
Transcribed Image Text:
(a) (b) Formaldehyde Methanethiol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
a The electrostatic potential map shows that the formaldehyde o...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the stronger acid, p-methoxybenzoic acid or p-(methylthio) benzoic acid?Explain. Anisole (CGH5OCH3)...
-
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions?...
-
The molecular electrostatic potential maps for LiH and HF are shown here. Does the apparent size of the hydrogen atom (shown as a white sphere) tell you whether it is an electron acceptor or an...
-
Many areas have attempted to increase the amount of recycled waste lubricating oil by requiring service stations to serve as collection centers or by instituting deposit-refund systems. On what...
-
How can asset allocation help you build an investment program to reach your financial goals?
-
Bank of America Corporation (BAC) and Wells Fargo & Company (WFC) are two large financial services companies. The following data (in millions) were taken from a recent years financial statements for...
-
For the CPPI formula (6.31), demonstrate that the strategy will breach the bond floor if S(ti)/S(ti1)1 < 1/m. Derive the value of an ATM put on such a strategy if m = 1.
-
Kee Company accumulates the following adjustment data at December 31. Indicate (a) The type of adjustment (prepaid expense, accrued revenue, and so on), (b) The status of accounts before adjustment...
-
- Employees salaries are $1,800,000, from this amount: $450,000 are income taxes; $71,000 are CPP contributions; and $35,000 are EI contributions. - As Employers we also have to contribute an equal...
-
Consider the weather Example 11.5.1, discussed earlier. The meteorologists prediction record over the past 15 days is as follows: (a) Assuming a uniform distribution for the states of nature, obtain...
-
The following structure represents the carbocation intermediate formed in the addition reaction of HBr to two different alkenes. Draw the structures ofboth.
-
Look at the following energy diagram: (a) Is ?G? for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are...
-
In the charging process described above, determine. (a) The change of entropy of refrigerant in the tank. (b) The entropy generation by the device and its surroundings. Assume the surrounding...
-
1. State the difference between lists and tuples in Python programming. 2. Explain why Python is an Interpreted Language
-
1. How does Python handle memory? 2. Python's ternary operators: how do they work? 3. How is Python's multithreading implemented
-
In a relational database, explain the difference between Inner join & Outer join. Provide an example query for each and describe the result set produced by each query.
-
Of the following, which method is a likely solution to successfully finish a project that is near completion but may be lagging? A. Ask for additional time to produce the deliverables. B. Ask for...
-
CRUZ, INC. Comparative Balance Sheets December 31, 2015 CRUZ, INC. Income Statement For Year Ended December 31, 2015 Required Use the indirect method to prepare the cash provided or used from...
-
Delocalized molecular orbitals are found in (a) H 2 ; (b) HS - ; (c) CH 4 ; (d) CO 3 2- .
-
Provide a detailed mechanism for each of the following reactions. Include contributing resonance structures and the resonance hybrid for the arenium ion intermediates. (a) (b) (c) HNO HSO NO2 Br Bra,...
-
Provide a detailed mechanism for the following reaction. H,SO + H2O
-
One ring of phenyl benzoate undergoes electrophilic aromatic substitution much more readily than the other. (a) Which one is it? (b) Explain your answer.
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


