In each case, give the structure of a compound with the indicated formula that would give the
Question:
In each case, give the structure of a compound with the indicated formula that would give the following diol in a LiAlH4 reduction followed by protonolysis.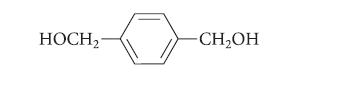
(a) C8H6O3
(b) C8H6O4
Transcribed Image Text:
HOCH2 -CH₂OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a The compound must contain three oxygens and two degrees of unsaturation in addition to ...View the full answer

Answered By

Subash Murugaih
I am leading expert in this web site couple of years and My clients are much happy with my works and services.
4.60+
309+ Reviews
539+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structure of a compound with the indicated formula that would give the following diol in a LiAlH4 reduction followed by protonolysis. C8H6O4 HOCH,- -CH,OH
-
Give the structure of a compound that satisfies each of the following criteria. (a) A compound C3H7ON that liberates ammonia on treatment with hot aqueous KOH (b) A compound that gives equal amounts...
-
Give the structure of a compound that fits each description. (There may be more than one correct answer for each.) (a) A chiral primary amine C4H7 N with no triple bonds (b) A chiral primary amine...
-
List the following classifications of accounts in all of the columns in which they appear on the work sheet, with the exception of the Adjustments columns. (Example: Assets) Assets ...................
-
Matthews Delivery Service, Inc., completed the following transactions during its first month of operations for January 2012: a. Matthews Delivery Service, Inc., began operations by receiving $6,000...
-
Why is Rent Expense a debit and Service Revenues a credit?
-
What benchmark would be most suitable? Perhaps the best benchmark to use would be the returns made by similar businesses operating in the same industry over the same period of time.
-
Ban Vallew has a son, Katt, by a previous marriage. Bans ex-wife has custody of Katt. Katt Vallew has a history of emotional problems, for which he has seen a psychiatrist for several years. This...
-
Tamarisk, Inc. collected $16920 in May of 2016 for 4 months of service which would take place from October of 2016 through January of 2017. The revenue reported from this transaction during 2016...
-
Give the structures of the products formed when (a) Chloroacetic acid and (b) P-chloro ben zoic acid react with P 2 O 5 .
-
Give the structure of the acid chloride formed in each of the following transformations. (a) Sodium ethanesulfonate + PCl 5 (b) Benzoic acid + SOCl 2 (c) P-toluenesulfonic acid 1 excess...
-
The inclined-tube manometer shown has \(D=76 \mathrm{~mm}\) and \(d=8 \mathrm{~mm}\), and is filled with Meriam red oil. Compute the angle, \(\theta\), that will give a \(15-\mathrm{cm}\) oil...
-
Classic Auto Parts sells new and used auto parts. Although a majority of its sales are cash sales, it makes a significant amount of credit sales. During 2012, its first year of operations, Classic...
-
The following information is available for Market Inc. and Supply Inc. at December 31, 2012: Required a. What is the accounts receivable turnover for each of the companies for 2012 ? b. What is the...
-
Buck Novak, the chief executive officer of Novak Corporation, has assembled his top advisers to evaluate an investment opportunity. The advisers expect the company to pay \($400,000\) cash at the...
-
Verify the log-likelihood in equation (16.4) for the Tobit model. In L = = In { 1-0 (x-di)} 1:y=di 122. + (y; - x) 02 (16.4) i:y;>di
-
Milo Company is considering the purchase of new equipment for its factory. It will cost \($250,000\) and have a \($50,000\) salvage value in five years. 1 he annual net income from the equipment is...
-
Compute the singular values and condition numbers of the 2 2, 3 3. and 4 4 Hilbert matrices. What is the smallest Hilbert matrix with condition number larger than 106?
-
The process of collaborative goal setting by a manager and subordinate, the extent to which goals are accomplished is a major factor in evaluating and rewarding the subordinate's performance. It is...
-
When 1,3,5-cycloheptatriene reacts with one molar equivalent of bromine at 0 8C, it undergoes 1,6 addition. (a) Write the structure of this product.
-
The cyclopentadienyl cation is apparently antiaromatic. Explain what this means in terms of the p-electron energies of a cyclic and an open-chain compound.
-
In 1967 R. Breslow (of Columbia University) and co-workers showed that adding SbCl5 to a solution of 3-chlorocyclopropene in CH2Cl2 caused the precipitation of a white solid with the composition C3H3...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


