In the conversion shown in Fig. P18.84, the DielsAlder reaction is used to trap a very interesting
Question:
In the conversion shown in Fig. P18.84, the Diels–Alder reaction is used to trap a very interesting intermediate by its reaction with anthracene. From the structure of the product, deduce the structure of the intermediate. Then write a mechanism that shows how the intermediate is formed from the starting material.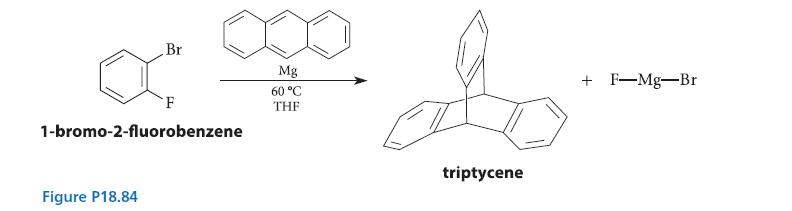
Transcribed Image Text:
Br Figure P18.84 F 000 do Mg 60 °C THE triptycene 1-bromo-2-fluorobenzene + F-Mg-Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Deduce the structure of the very interesting intermediate by mentally imagini...View the full answer

Answered By

Felix Mucee
I am a detailed and thorough professional writer with 5 years of administrative experience- the last 2 years in academic writing and virtual office environment. I specialize in delivering quality services with respect to strict deadlines and high expectations. I am equipped with a dedicated home office complete with a computer, copier/scanner/fax and color printer.
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past five years. I can bring value to your business and help solve your administrative assistant issues.
4.70+
13+ Reviews
33+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the conversion shown in Fig. P18.77, the Diels-Alder reaction is used to trap a very interesting intermediate by its reaction with anthracene. From the structure of the product, deduce the...
-
A liquid-phase biological reaction is used to produce an intermediate chemical for use in the pharmaceutical industry. The reaction occurs in a large, well-stirred bioreactor that is illustrated in...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Using the SEDAR database, find the most recent annual reports for two Canadian retailers (e.g., Loblaw, Rona, Danier Leather). Required: a. Based on the information provided in the companies audited...
-
Consider the facts presented in the following table for Tropical View, Inc.: Requirements 1. Complete the table by filling in the missing values. 2. Prepare one journal entry for each situation for...
-
On December 31, 2013, WOR Productions reported $100,000 of contributed capital and $20,000 of retained earnings. During 2014, the company had the following transactions. Prepare a statement of...
-
Explain how exchange rates are determined. LO.1
-
On January 3, 2014, Speedway Delivery Service purchased a truck at a cost of $ 65,000. Before placing the truck in service, Speedway spent $ 4,000 painting it, $ 2,500 replacing tires, and $ 8,000...
-
14. A European put option on Google stock costs $32. It expires in 0.5 years and has a strike price of $800. Google's stock price is $870. The risk-free rate is 1.7% (continuously compounded). a....
-
Propose a structure for the product A obtained in the following oxidation of 2,4,6-trimethylphenol. (Compound A is an example of a rather unstable type of compound called generally a quinone...
-
Explain why the dipole moment of 4-chloronitrobenzene (2.69 D) is less than that of nitrobenzene (3.99 D), and the dipole moment of p-nitroanisole (1-methoxy-4-nitrobenzene, 4.92 D) is greater than...
-
Based on the data presented in Exercise 5-8, journalize the closing entries.
-
Which topics do you see as being most relevant to your current job or the job you will seek to obtain once you have earned your degree? How so ? In which ways has this course Commercial Law changed...
-
Directions Answer the following reflective questions: There do exist examples of business organizations following principles of behavior that are not entirely self-serving, but rather, are pursuing...
-
10 Count scallops cost $12.97 per pound. How much do they cost for each? A Wagyu Beef New York Strip costs $14 per pound and weighs 15 pounds. The useable yield is 12.5 pounds. How many 12 ounce...
-
How do coordinating agencies differ in a crisis, disaster, and an emergency ?Explain
-
How do we manage and respond to customer feedback and reviews to maintain a positive brand reputation? Explain with the help of examples.
-
Show that each eigenspace of an n n matrix A is an invariant subspace, as defined in Exercise 7.4.32. Exercise 7.4.32 The subspace W of a vector space V is said to be an invariant subspace under the...
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
Predict the products from the following reactions. (a) (b) (c) (d) (1) 2 exces) NaoH, Ho (2) H,o KOH 2 HOEIOH KOH H2O/EtOH (1) LDA (1.1 equiv) (3) H2o
-
The mandibular glands of queen bees secrete a fluid that contains a remarkable compound known as "queen substance." When even an exceedingly small amount of the queen substance is transferred to...
-
What products would you expect to obtain from each of the following crossed Claisen condensations? (a) (b) Ethyl propanoate+ (1) NaOEt (2) H,o yl oxalate (1) NaOEt Ethyl acetate ethyl formate (2) H,O
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


