Name the following compounds. Ignore double-bond stereochemistry. (a) Me Me (b) CH3CHCH=CHCHCHCH3
Question:
Name the following compounds. Ignore double-bond stereochemistry.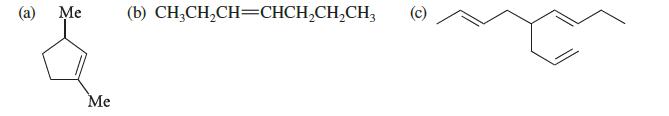
Transcribed Image Text:
(a) Me Me (b) CH3CH₂CH=CHCH₂CH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a 13dimethy...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Name the following compounds by IUPAC rules: a. b. H-C CH,CH-CH
-
Name the following compounds by the IUPAC system: a. CH3CH=C(CH2CH2CH3)2 b. (CH3)2CHCH"CHCH3 c. g. CH3-C-C-CH-CH, h. k.
-
Name the following compounds and assign oxidation states to the halogens in them: (a) Fe(ClO3)3 (b) HClO2 (c) XeF6 (d) BrF5 (e) XeOF4 (f) HIO3.
-
What are the final values of x and y? int x= 1, y=6; if(x>=1) if(y> 5){ X=X+2; y=y+2; } else{ X=X-1; y=y-1; } X=X+1; y=y+1;
-
If you are persuaded by Yeagles arguments, please answer the following questions:
-
(a) Consider the AF3 molecules in Exercise 9.29.Which of these will have a nonzero dipole moment? Explain. (b) Which of the AF4 molecules in Exercise 9.30 will have a zero dipole moment? In Exercise...
-
Why can the end of a project be stressful for many of the project stakeholders? AppendixLO1
-
Is there a relationship between the race of violent offenders and their victims? Data from the U.S. Department of Justice (Expanded Homicide Data Table 6, 2011) are presented below. a. Let's treat...
-
Harrison exchanges his apartment complex for Hanna's farm, and the exchange qualifies as a like-kind exchange. Harrison's adjusted basis for the apartment complex is $610,000 and the complex is...
-
What is the configuration of the following stereoisomer of 3-methyl-2-pentene? (The numbers are for reference in the solution.) H3C 2 3 c=c CH3 CHCH3
-
Give the structure for each of the following: (a) 2-methylpropene (b) 4-methyl-1,3-hexadiene (c) 1-isopropenylcyclopentene (d) 5-(3-pentenyl)-1,3,6,8-decatetraene
-
In 2019, you gave $15,000 worth of stock to your best friend. In 2020, the stock is valued at $25,000. a. What was the gift tax in 2019? b. What is the total amount removed from your estate in 2020?...
-
Saskatchewan Soy Products (SSP) buys soy beans and processes them into other soy products. Each tonne of soy beans that SSP purchases for $300 can be converted for an additional $200 into 500 lbs of...
-
Pharoah Acres sponsors a defined-benefit pension plan. The corporation's actuary provides the following information about the plan: January 1, 2025 December 31, 2025 Vested benefit obligation $510...
-
Company panther is compelled to pick between two machines An and B. The two machines are planned in an unexpected way, yet have indistinguishable limit and do the very same work. Machine A costs...
-
On April 30, 2023, a company issued $600,000 worth of 5% bonds at par. The term of the bonds is 9 years, with interest payable semi- annually on October 31 and April 30. The year-end of the company...
-
Identify the following; MethodBodyReturn statementReturn typeParameter Look at this example we saw in our Methods lesson: public double findTheArea (double length, double w idth) { double area =...
-
Watch the following structures with their descriptions: A. sacs that contain enzymes that catalyze a variety of specific (1) Golgi apparatus biochemical reactions 2) mitoxhondia Bsctures on which...
-
On April 29, 2015, Auk Corporation acquires 100% of the outstanding stock of Amazon Corporation (E & P of $750,000) for $1.2 million. Amazon has assets with a fair market value of $1.4 million (basis...
-
Look at Figure, and estimate the percentages of axial and equatorial conformers present at equilibrium inbromo-cyclohexane. Energy difference (kcal/mol) 2 100 More stable isomer 80 60 20 Less stable...
-
Draw the most stable chair conformation of the following molecules, and estimate the amount of strain in each: (a) trans-1-Chloro-3-methylcyclohexane (b) cis-1-Ethyl-2-rnethylcyclohexane (c)...
-
Identify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (yellow green ? CI):
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


