Only one of the following three alkyl halides can be prepared as the major product of the
Question:
Only one of the following three alkyl halides can be prepared as the major product of the addition of HBr to an alkene. Which compound can be prepared in this way? Explain why the other two cannot be prepared in this way.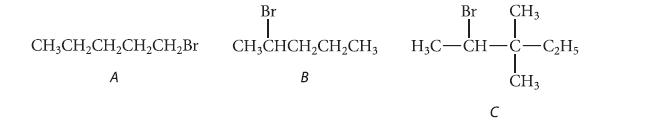
Transcribed Image Text:
CH3CH₂CH₂CH₂CH₂Br A Br I CHỊCHCH,CH,CH, B Br I CH3 H3C-CH-C-C₂H5 I CH3 C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
If these alkyl halides could be prepared from alkenes the alkene sta...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. Hannah is applying for a life policy on her girlfriend Sarahs life. The policy is $500,000 and carries a large premium. Hannah is the main earner, so she is concerned about not being able to pay...
-
MCQ questions: 1. An increase in oil prices, such as the oil shocks in the 70s, lead to _______ thereby causing ________ a movement along the AS curve; cost-push inflation a leftward shift in the AS...
-
Four million infants in the United States consume 80 million cases of jarred baby food annually, representing a domestic market of $865 million to $1 billion. The baby food market is dominated by...
-
What is the Antebellum Interregional growth hypothesis?
-
Was the phrase defamatory, or was it deliberate exaggeration that no reasonable person would take literally?
-
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation-anion distances are 2.01 Ã (Li-F), 2.82 Ã (Na-Cl), 3.30 Ã (K-Br), and 3.67 Ã (Rb-I), respectively. (a)...
-
Five players on a basketball team must each choose a player on the opposing team to defend. In how many ways can they choose their defensive assignments?
-
The following relative frequency ogive represents the lengths of a random sample of tornadoes in the United States. (a) What is the class width? (b) Approximately 92% of all tornadoes are less than...
-
Question 2 1 pts As part of its capital structure as of December 31, 2018, Lucas Company has 1,000 shares of cumulative, convertible preferred stock. Each share is convertible into 5 shares of common...
-
Draw curved-arrow mechanisms and transition-state structures for each of the following two reactions. Each reaction occurs as a single step. (a) CHCH-Br: + FCH3 CHCH-CH3 + :Br: (b) (CH3)3C-Br:...
-
Which of the following carbocations is likely to rearrange? If rearrangement occurs, give the structures of the rearranged carbocations. @ H I + CH3 (b) CH3 CH3 T T CH3CH-C-CH3 + O
-
Countrytime Studios is a recording studio in Nashville. The studio budgets and applies overhead costs on the basis of production time. Countrytime's controller anticipates 10,000 hours of production...
-
5. Group the majors and construct a relative frequency distribution with a circle graph with this information. Program of Study bus AA Bus Eng AS Nur mech AA Nur AA AS Nur AS Nur AA DE AS AA AS AA AA...
-
1 2 Let f (x) = and g(x) = +4. x-3 a. Find and simplify (go f) (x). (gf) (x) = b. Find the restriction for the domain of (go f) (x) Domain restriction: x + each value using comma.) (If there's more...
-
The Casings Plant of Wyoming Machines makes plastics shells for the company's calculators. (Each calculator requires one shell.) For each of the next two years, Wyoming expects to sell 660,000...
-
(f) A windowless office is to be illuminated for 15 hours per day, for 6 days per week, for 50 weeks per year. The floor is 20 m long and 12 m wide. An overall illumination of 450 Lux is to be...
-
Give the series of basic transformation matrices that transform the wedge below so that it can sit "on top of" the following parallelepiped to form a simple "house" with the ridge line of the roof...
-
Describe how negative feedback involving a rate-limiting enzyme controls a metabolic pathway.
-
AB CORPORATION ISSUED THE FOLLOWING 850 COMMON STOCKS PAR VALUE P100 750 PARTICIPATING PREFERRED STOCKS PAR VALUE P100 AT 3% AB CORPORATION DECLARED P100,000.00 DIVIDEND IN 2022.
-
Give IUPAC names for the followingcompounds: (b) (a) The three isomers of C5H12 CH CH;CH-CHH CH (d) (c) CH CH (CH)2CHCH2CH3 (CH3)3CH-H2C CH
-
Draw structures corresponding to the following IUPAC names: (a) 3, 4-Dimethylnonane (b) 3-Ethyl-4, 4-dimethylheptane (c) 2, 2-Dimethyl-4-propylocatane (d) 2, 2, 4-Trimethylpentane
-
Name the eight 5-carbon alkyl groups you drew in Problem 3.7.
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


