Which of the following carbocations is likely to rearrange? If rearrangement occurs, give the structures of the
Question:
Which of the following carbocations is likely to rearrange? If rearrangement occurs, give the structures of the rearranged carbocations.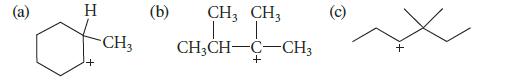
Transcribed Image Text:
@ H I + CH3 (b) CH3 CH3 T T CH3CH-C-CH3 + O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Rearrangement will occur when a carbocation can rearrange b...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Each of the following carbocations can rearrange to a more stable ion. Propose structures for the likely rearrangement products. H, (a) CH3CH2CH2CH2* (b) CH3CHCHCH3 CH CH CH2* (c)
-
The following questions are adapted from a variety of sources including questions developed by the AICPA Board of Examiners and those used in the Kaplan CPA Review Course to study property, plant,...
-
Each of the following carbocations has the potential to rearrange to a more stable one. Write the structure of the rearranged carbocation. (a) CH3CH2CH2+ (b) (c) (d) (CH3CH2)3CCH2+ (e) (CH32CHCHCHa...
-
1. Consider a local department store which only sells jeans (J) and sweaters (S). Jeans cost $20 and sweaters cost $30. For each of the examples below, graph the budget constraint our consumer faces,...
-
Why was Konop particularly eager to keep unauthorized people out of his web site?
-
Make a simple sketch of the shape of the main part of the periodic table, as shown. (a) Ignoring H and He, write a single straight arrow from the element with the smallest bonding atomic radius to...
-
A literary magazine editor must choose 4 short stories for this months issue from 17 submissions. In how many ways can the editor choose this months stories?
-
What is the net present value of an investment that costs $75,000 and has a salvage value $45,000? The annual profit from the investment is $15,000 each year for 5 years. The cost of capital at this...
-
Waymouth Manufacturing operates a contract manufacturing plant located in Dublin, Ireland. The plant provides a variety of electronics products and components to consumer goods manufacturers around...
-
Only one of the following three alkyl halides can be prepared as the major product of the addition of HBr to an alkene. Which compound can be prepared in this way? Explain why the other two cannot be...
-
In each case, give two different alkene starting materials that would react with HBr to give the compound shown as the major (or only) addition product. (a) Br (b) Me Br
-
For each polynomial function, (a) list all possible rational zeros, (b) find all rational zeros, and (c) factor (x) into linear factors. (x) = 24x 3 + 40x 2 - 2x - 12
-
Matthew Kennedy of Urbana, Ohio, is single and has been working as an admissions counselor at a university for five years. Matthew owns a home valued at $250,000 on which he owes $135,000. He has a...
-
Question: A group of employees of Unique Services will be surveyed about a new pension plan. In-depth interviews with each employee selected in the sample will be conducted. The employees are...
-
On January 1, 2020, the following accounts appeared in the general ledger of Ace's Repair Shop: Cash P10,500 Accounts receivable 8.400 Furniture 12,600 Repair Equipment 54,000 Accounts Payable 22,000...
-
Your maths problem x+3x-3 Find solutions on the web Q +1 XII
-
5. Data for the payroll for the Dos Company for the month of April are shown below: Total gross earnings Social security taxes withheld Phil Health taxes withheld Employees income tax withheld...
-
Consider the following DNA sequence: CATGTGTAGTCTAAA a. Write the sequence of the DMA strand that would be repeated from this one b. Write the sequence of the RMA molecule that would be transcribed...
-
Smiths Family Fashions implemented a balanced scorecard performance measurement system several years ago. Smiths is a locally owned clothing retailer with fashions for men, women, teens, and...
-
Give the IUPAC name for the following hydrocarbon, and convert the drawing into a skeletal structure.
-
Make a graph of potential energy versus angle of bond rotation for propane, and assign values to the energy maxima.
-
Consider 2-methylpropane (isobutene). Sighting along the C2-C1 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. (c)...
-
As a long-term investment at the beginning of the 2018 fiscal year, Florists International purchased 25% of Nursery Supplies Inc.'s 18 million shares for $66 million. The fair value and book value of...
-
Javier is currently paying $1,200 in interest on his credit cards annually. If, instead of paying interest, he saved this amount every year, how much would he accumulate in a tax-deferred account...
-
Your company is considering the purchase of a fleet of cars for $195,000. It can borrow at 6%. The cars will be used for four years. At the end of four years they will be worthless. You call a...

Study smarter with the SolutionInn App


