Outline a malonic ester synthesis of the following carboxylic acid: CH3 COH 2-methylheptanoic acid
Question:
Outline a malonic ester synthesis of the following carboxylic acid: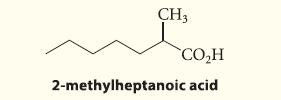
Transcribed Image Text:
CH3 CO₂H 2-methylheptanoic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Using the analysis in the text identify the acetic acid unit in the carboxylic acid T...View the full answer

Answered By

Gilbert Chesire
I am a diligent writer who understands the writing conventions used in the industry and with the expertise to produce high quality papers at all times. I love to write plagiarism free work with which the grammar flows perfectly. I write both academics and articles with a lot of enthusiasm. I am always determined to put the interests of my customers before mine so as to build a cohesive environment where we can benefit from each other. I value all my clients and I pay them back by delivering the quality of work they yearn to get.
4.80+
14+ Reviews
49+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What alkyl bromide(s) should be used in the malonic ester synthesis of each of the following carboxylic acids? a. Propanoic acid b. 2-methylpropanoic acid c. 3-phenylpentanoic acid d....
-
a. What carboxylic acid would be formed if the malonic ester synthesis were carried out with one equivalent of malonic ester, one equivalent of 1,5-dibromopentane, and two equivalents of base? b....
-
Outline all steps in a malonic ester synthesis of each of the following: (a) pentanoic acid, (b) 2-methylpentanoic acid, (c) 4-methylpentanoic acid.
-
Suppose Targets stock has an expected return of 20% and a volatility of 40%, Hersheys stock has an expected return of 12% and a volatility of 30%, and these two stocks are uncorrelated. a. What is...
-
Shine King Cleaning has decided that, in addition to providing cleaning services, it will sell cleaning products. During December, Shine King completed the following transactions: Dec 2 Purchased 600...
-
Recently, Pfizer and Allergan-the makers of Viagra and Botox, respectively-initiated a $160 billion merger. Pharmaceutical companies tend to spend a greater percentage of sales on R&D activities than...
-
Discuss the relationship between government intervention and protectionism. LO.1
-
A ticket from Indianapolis to Orlando on Deleast Airlines sells for $150. The plane can hold 100 people. It costs Deleast $8000 to fly an empty plane. Each person on the plane incurs variable costs...
-
A budget plan that shows the budgeted costs of direct materials to be purchased by a company to satisfy the budgeted production for the budget period is called a: A. Capital expenditure budget B....
-
Explain why the following compound does not undergo base-catalyzed exchange in D 2 O even though it has an a-hydrogen. does not exchange in base/DOH O CH3 CH3
-
Write a mechanism involving an enolate ion intermediate for the reaction shown in Eq. 22.6. Explain why only the a-hydrogens are replaced by deuterium. H H H L DO/dioxane Et3N: (a base) heat, 48 h D...
-
When does a not-for-profit organization record donated services?
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
(b) Explain why the two systems have the same phase portraits, but the direction of motion along the trajectories is reversed. (c) Apply time reversal to the system(s) you derived in Exercise 9.1.1....
-
Tarick Toys Company manufactures video game consoles and accounts for product costs using process costing. The following information is available regarding its June inventories. The following...
-
Write a mechanism that accounts for the following reaction: OH HA + HOH
-
Propose a reasonable mechanism for the following reaction. cat. H2SO4 OH- C3
-
Propose a reasonable mechanism for the following reaction. OH
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


