Explain why the following compound does not undergo base-catalyzed exchange in D 2 O even though it
Question:
Explain why the following compound does not undergo base-catalyzed exchange in D2O even though it has an a-hydrogen.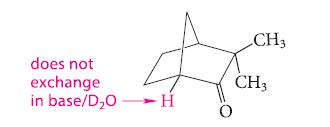
Transcribed Image Text:
does not exchange in base/D₂OH O CH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Exchange does not occur because the orbital containing the unshared electron pair on the acarbo...View the full answer

Answered By

John Kago
Am a processional practicing accountant with 5 years experience in practice, I also happens to have hands on experience in economic analysis and statistical research for 3 years. am well conversant with Accounting packages, sage, pastel, quick books, hansa world, etc, I have real work experience with Strata, and SPSS
4.70+
31+ Reviews
77+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following compound is not aromatic even though it has 4n + 2 electrons in a continuous cyclic array. Explain why this compound is not aromatic.
-
Explain why the following alkyl halide does not undergo a substitution reaction, regardless of the conditions under which the reaction is run: Cl
-
The bicycle ketone shown below does not undergo aldol self-condensation even though it has two ? hydrogen atoms. Explain.
-
Suppose that General Motors Acceptance Corporation issued a bond with 10 years until maturity, a face value of $1000, and a coupon rate of 7% (annual payments). The yield to maturity on this bond...
-
G Wholesale Company began the year with inventory of $6,000. During the year, G purchased $97,000 of goods and returned $6,200 due to damage. G also paid freight charges of $1,500 on inventory...
-
You are the manager of a large but privately held online retailer that currently uses 17 unskilled workers and 6 semiskilled workers at its warehouse to box and ship the products it sells online....
-
There has been much opposition to the Trans-Pacific Partnership (TPP). For a sampling of arguments against this proposed pact, visit www.globalexchange.org, www.citizenstrade. org, and...
-
Brady is hired in 2015 to be the accountant for Anderson Manufacturing, a private company. At the end of 2015, the balance of Accounts Receivable is $29,000. In the past, Anderson has used only the...
-
i need some help please The following information is available for Lock-Tite Company, which produces special-order security products and uses a job order costing system. April 30 May 31 $ $49,800...
-
Sketch the proton NMR spectrum of 2-butanone, and explain how this spectrum would change if the compound were treated with D 2 O and a base.
-
Outline a malonic ester synthesis of the following carboxylic acid: CH3 COH 2-methylheptanoic acid
-
Which is not part of the statistical section of a CAFR? 1. List of principal officials. 2. Debt capacity information. 3. Demographic and economic information. 4. Financial trends information.
-
Suppose a small flashlight bulb is on the bottom of the bathtub of Problem 19, directly under the toy boat. When this bulb is lit and the ceiling light is turned off, how does the size of the shadow...
-
Draw a scatter diagram and find \(r\) for the data shown in each table in Problems 25-30. X 85 90 y 80 40 100 30 102 28 105 25
-
Rothera Point Utilities (RPU) provides customers with 7 million megawatt-hours (MWh) of electricity each year. RPU operates three different generation facilities to meet this demand: the Rothera...
-
Explain the components of the path evaluation function f(node) used by A*. Do you think it is the best evaluation function that could be used? To what kinds of problems might it be best suited? And...
-
Celvin FoodStuff operates a chain of mini conve- nience stores in downtown city settings, offering beverages, snack food, and some fresh food items to passing pedestrian traffic. A typical Celvin...
-
(b) How are the solution trajectories of the two systems related? Au.
-
Refer to the information from Exercise 22-19. Use the information to determine the (1) Weighted average contribution margin , (2) Break-even point in units, and (3) Number of units of each product...
-
Provide the reagents necessary to accomplish the following syntheses. (a) (b) (c) (d) MeO MeO SEt SEt
-
Provide reagents that would accomplish the follwing syntheses. (a) (b) HO Glycerol Epichlorohydrin
-
Write structures for compounds A-J showing stereochemistry where appropriate. (a) What is the stereochemical relationship between A and C? (b) (c) What is the stereochemical relationship between H...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


