Outline a synthesis of pentanoic acid (valeric acid) from 1-butanol. CH3CHCHCH-OH 1-butanol CH3CHCHCH-C-OH pentanoic acid
Question:
Outline a synthesis of pentanoic acid (valeric acid) from 1-butanol.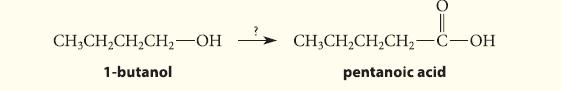
Transcribed Image Text:
CH3CH₂CH₂CH₂-OH 1-butanol CH3CH₂CH₂CH₂-C-OH pentanoic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
A new carboncarbon bond must be formed at some point in this synthe...View the full answer

Answered By

Aqib Parvej
I am teaching since my graduation time so I have teaching experience of about 5 years and in these years I learn to teach in the best and interesting way .
4.80+
20+ Reviews
41+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Fatty acids containing an even number of carbon atoms are readily obtained from natural sources, but those containing an odd number of carbons are relatively rare. Outline a synthesis of the rare...
-
Using decarboxylation reactions outline a synthesis of each of the following from appropriate starting materials: (a) 2-Hexanone (b) 2-Methylbutanoic acid (c) Cyclohexanone (d) Pentanoic acid
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) 1-cyclohexyl-2-methyl-2-prupanol from bromocyclohexane (b) PhNHCH2CH2CH(CH3)2...
-
Since perpetuity payments continue forever, how can a present value be computed? Why isnt the present value infinite?
-
Refer to the facts presented in Exercise 5-15. Requirement 1. Journalize the transactions of the seller, Slater Diamonds. Slaters cost of goods sold was 45% of the sales price. Explanations are not...
-
Refer to Cornerstone Exercise 5-6 for data. Now assume that the Pineapple Pen Company uses the sequential method to allocate support department costs to the producing departments. Maintenance is...
-
Can you figure out the possible reasoning behind this suggestion? Do you agree with such a suggestion?
-
Hoffman Containers manufactures a variety of boxes used for packaging. Sales of its Model A20 box have increased significantly to a total of 480,000 A20 boxes. Hoffman has enough existing production...
-
The following data concerning the retail inventory method are taken from the financial records of Sheridan Company. Cost Retail Beginning inventory $ 192000 $ 283000 Purchases 905000 1160000...
-
Give a structure for each of the following compounds. (a) 5-cyanopentanoic acid (b) Isopropyl valerate (c) Ethyl methyl malonate (d) Cyclohexyl acetate (e) N,N-dimethylformamide (f) g-valerolactone...
-
A water-insoluble hydrocarbon A decolorizes a solution of Br 2 in CH 2 Cl 2 . The base peak in the EI mass spectrum of A occurs at m/z = 67. The proton NMR of A is complex, but integration shows that...
-
The source voltage in Figure 554 is 100 V. How much voltage does each of the three meters read? + 100 V V1 FIGURE 5-54 R www R + V2 R3 V3
-
The following post-closing trial balance was drawn from the accounts of Spruce Timber Co. as of December 31, 2011. Transactions for 2012 1. Acquired an additional \(\$ 10,000\) cash from the issue of...
-
Bankers Trust (BT) was one of the most powerful and profitable banks in the world in the early 1990s. Under the stewardship of chairman Charles Sanford Jr., it had transformed itself from a staid...
-
Hammond Inc. experienced the following transactions for 2011, its first year of operations: 1. Issued common stock for \(\$ 80,000\) cash. CHECK FIGURES b. Net Income: \(\$ 62,520\) Total Assets:...
-
Following are the current prices and last years prices of a gallon of regular gas at a sample of 14 gas stations. Can you conclude that the median price is different now from what it was a year ago?...
-
A sample of nine men participated in a regular exercise program at a local gym. They were weighed both before and after the program. The results were as follows. Can you conclude that the median...
-
Write down all possible 3 3 Jordan matrices that have eigenvalues 2 and 5 (and no others).
-
Read the Forecasting Supply Chain Demand Starbucks Corporation case in your text Operations and Supply Chain Management on pages 484-485, then address the four questions associated with the...
-
Write the important resonance structures for each of the following: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) CH2 CH-Br NO2
-
Predict the products of the following reactions. (a) (b) (c) HBr 15 C HBr 40C hv, heat
-
Provide a mechanism that explains formation of the following products. CI HCI (concdCI+
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


