Predict the major product(s) of each of the following transformations. (a) Et C-CH + CHOH (solvent) *****
Question:
Predict the major product(s) of each of the following transformations.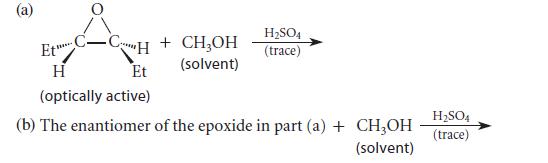
Transcribed Image Text:
(a) Et C-CH + CH₂OH (solvent) ***** H Et (optically active) H₂SO4 (trace) H₂SO4 (b) The enantiomer of the epoxide in part (a) + CH3OH (trace) (solvent)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a b The nucleophilic reaction of methanol on the protonated epoxide occurs with inversion to give 3...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product from each of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH NH2, cat. HA N-H, cat. HA NH2 cat. HA PPha (1) HS SH (2) Raney Ni, H2 0 CH2PPha (excess) O. O...
-
Predict the major product from each of the following reactions. (a) (b) (c) (d) (e) (f) OH SOC, pyr OH HBr NaNH2 OH OH (1) TsCI, pyr (2) EtSNa Nal, H2SO OH
-
Predict the major product from each of the following reactions. (a) (b) (c) (d) (e) HO OH NH2 HA (cat.) (1) HCN (2) LiAIH4 (3) H2o mCPBA
-
Question 13 Case Study - Little Pear Administration Pty Ltd (LPA) You are the payroll officer for Little Pear Administration Pty Ltd (LPA). Debra Foy is a company employee and has approached you with...
-
Why dont the additions and deductions from the bank balance on a bank reconciliation require adjustment by the company?
-
Hayward Industries manufactures dining chairs and tables. The following information is available: Perform the following analyses for these two components of overhead: Compute total machine setups and...
-
2-5. What is the difference between a marketing dashboard and a marketing metric?
-
Petroleum Research, Inc. (A), and Extraction International, Inc. (B), have each developed a new extraction procedure that will remove metal and other contaminants from used automotive engine oil. The...
-
Prepare a contribution margin income statement for the next year assuming sales decrease by 4%. Prepare a contribution margin income statement for the next year assuming sales decrease by 4%
-
From what alkene could each of the following glycols be prepared by the OsO 4 or KMnO 4 method? OH I CH3CHOCHCHCHCHOH (b) OH -CHOH (c) meso-4,5-octanediol (d) ()-4,5-octanediol
-
Explain each of the following facts with a mechanistic argument. (a) When butyl methyl ether (1-methoxybutane) is treated with HI and heat, the initially formed products are mainly methyl iodide and...
-
A small machine tool of mass \(100 \mathrm{~kg}\) operates at \(600 \mathrm{rpm}\). Find the static deflection of an undamped isolator that provides 90 percent isolation.
-
Brian is considering increasing the length of the cryptographic keys used by his organization. If he adds 8 bits to the encryption key, how many more possible keys will be added to the key space for...
-
Business law SECHON A [100 Marks] Read the scenario below then answer the questions that follow. Contracts are of critical importance especially in daily commercial and business transactions....
-
You may assume that the production costs to the winery are the same for each of the possible wines, despite the differences in volumes with some of the possible wines. Thus maximizing revenue will be...
-
You encounter a split system that uses R-22 refrigerant and observe the following refrigeration parameters from the unit's control display. The unit is operating in cooling mode. Suction pressure:...
-
A refrigerant at -20C is flowing through a 4" schedule 40 carbon steel pipe (inner diameter 102 mm, outer diameter 114 mm); the heat transfer coefficient for the refrigerant is 2500 W/m/K. It is...
-
An isosceles triangle is topped by a semicircle, as shown in Figure 18. Find a formula for the area A of the whole figure in terms of the side length r and angle t (radians). (We say that A is a...
-
Design an experiment to demonstrate that RNA transcripts are synthesized in the nucleus of eukaryotes and are subsequently transported to the cytoplasm.
-
Show the products you would expect to obtain from reaction of glyceryl trioleate with the following reagents: (a) Excess Br2 in CH2Cl2, (b) H2/Pd (c) NaOH/H2C (d) O3, then Zn/CH3CO2H (e) LiAlH4, then...
-
How would you convert oleic acid into the following substances? (a) Methyl oleate (b) Methyl stearate (c) Nonanal (d) Nonanedioic acid (e) 9-Octadccynoic acid (Stearolic acid) (f) 2-Bromostearic acid...
-
Cold-water fish like salmon are rich in omega-.3 fatty acids, which have a double bond three carbons in from the non carboxyl end of the chain and have been shown to lower blood cholesterol levels....
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


